
Column-based nucleic acid purification
Encyclopedia
Nucleic acid
Nucleic acids are biological molecules essential for life, and include DNA and RNA . Together with proteins, nucleic acids make up the most important macromolecules; each is found in abundance in all living things, where they function in encoding, transmitting and expressing genetic information...
purification
Purification
Purification is the process of rendering something pure, i.e. clean of foreign elements and/or pollution, and may refer to:* List of purification methods in chemistry* Water purification** Organisms used in water purification...
is a solid phase extraction
Solid phase extraction
Solid-phase extraction is a separation process by which compounds that are dissolved or suspended in a liquid mixture are separated from other compounds in the mixture according to their physical and chemical properties. Analytical laboratories use solid phase extraction to concentrate and purify...
method to quickly purify nucleic acids.
This method relies on the fact that the nucleic acid
Nucleic acid
Nucleic acids are biological molecules essential for life, and include DNA and RNA . Together with proteins, nucleic acids make up the most important macromolecules; each is found in abundance in all living things, where they function in encoding, transmitting and expressing genetic information...
may bind (adsorption
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
) to the solid phase (silica or other) depending on the pH and the salt content of the buffer, which may be a Tris-EDTA (TE)
TE buffer
TE buffer is a commonly used buffer solution in molecular biology, especially in procedures involving DNA or RNA. "TE" is derived from its components: Tris, a common pH buffer, and EDTA, a molecule that chelates cations like Mg2+...
buffer or Phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
buffer
Buffer solution
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. It has the property that the pH of the solution changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a...
(used in DNA microarray experiment
DNA microarray experiment
This is an example of a DNA microarray experiment, detailing a particular case to better explain DNA microarray experiments, while enumerating possible alternatives.# The two samples to be compared are grown/acquired...
s due to the reactive amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s).
Therefore, three stages are:
- The sampleSample (material)In general, a sample is a limited quantity of something which is intended to be similar to and represent a larger amount of that thing. The things could be countable objects such as individual items available as units for sale, or a material not countable as individual items. Samples of countable...
is added to the columnColumnA column or pillar in architecture and structural engineering is a vertical structural element that transmits, through compression, the weight of the structure above to other structural elements below. For the purpose of wind or earthquake engineering, columns may be designed to resist lateral forces...
and the nucleic acidNucleic acidNucleic acids are biological molecules essential for life, and include DNA and RNA . Together with proteins, nucleic acids make up the most important macromolecules; each is found in abundance in all living things, where they function in encoding, transmitting and expressing genetic information...
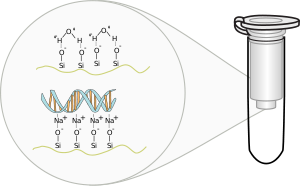
binds due to the lower pHPHIn chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
(relative to the silanolSilanolSilanol, also known as silyl alcohol, is a chemical with formula SiH3OH. It is the simplest silicon alcohol, and is a heavy, volatile, colorless, flammable liquid. At room temperature it is a polar liquid...
groups on the column) and saltSaltIn chemistry, salts are ionic compounds that result from the neutralization reaction of an acid and a base. They are composed of cations and anions so that the product is electrically neutral...
concentrationConcentrationIn chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
of the binding solutionSolutionIn chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
, which may contain bufferBuffer solutionA buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. It has the property that the pH of the solution changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a...
, a denaturing agentDenaturation (biochemistry)Denaturation is a process in which proteins or nucleic acids lose their tertiary structure and secondary structure by application of some external stress or compound, such as a strong acid or base, a concentrated inorganic salt, an organic solvent , or heat...
(such as guanidine hydrochloride), Triton X-100, isopropanol and a pH indicatorPH indicatorA pH indicator is a halochromic chemical compound that is added in small amounts to a solution so that the pH of the solution can be determined visually. Hence a pH indicator is a chemical detector for hydronium ions or hydrogen ions in the Arrhenius model. Normally, the indicator causes the... - The column is then washed (5 mM KPO4 pH 8.0 or similar, 80% EtOHEtohEtoh may refer to:*ETOH, when used in a medical context, is an abbreviation for ethanol . For instance, "ETOH 10g/day" denotes that the patient drinks 10 grams of alcohol a day on average....
) - The column can be eluted with buffer or simply water
Even prior to the major techniques
Nucleic acid methods
Nucleic acid methods are the techniques used to study nucleic acids .Purification*Phenol-chloroform extraction*minicolumn purification*RNA extractionQuantification*Abundance in weight: spectroscopic quantification...
employed today it was known that in the presence of chaotropic agents, such as sodium iodide
Sodium iodide
Sodium iodide is a white, crystalline salt with chemical formula NaI used in radiation detection, treatment of iodine deficiency, and as a reactant in the Finkelstein reaction.-Uses:Sodium iodide is commonly used to treat and prevent iodine deficiency....
or sodium perchlorate
Sodium perchlorate
Sodium perchlorate is the inorganic compound with the chemical formula NaClO4. It is the most soluble of the common perchlorate salts. It is a white crystalline, hygroscopic solid that is highly soluble in water and in alcohol...
, DNA binds to silica, glass particles
Soda-lime glass
Soda-lime glass, also called soda-lime-silica glass, is the most prevalent type of glass, used for windowpanes, and glass containers for beverages, food, and some commodity items...
or to unicellular algae
Algae
Algae are a large and diverse group of simple, typically autotrophic organisms, ranging from unicellular to multicellular forms, such as the giant kelps that grow to 65 meters in length. They are photosynthetic like plants, and "simple" because their tissues are not organized into the many...
called diatoms which shield their cell walls with silica. This property was used to purify nucleic acid using glass powder or silica beads under alkaline conditions. This was later improved used guanidinium thiocyanate
Guanidinium thiocyanate
Guanidinium thiocyanate is a chemical compound used as a general protein denaturant, being a chaotropic agent, although it is most commonly used in the extraction of DNA and RNA.Note: this compound may also be recognized as guanidine thiocyanate...
or guanidinium hydrochloride as the chaotropic agent. The use of beads was later changed to minicolumns.
- For explanation of how the silicon and DNA bind see separation by silica adsorption
See also
- Guanidinium thiocyanate-phenol-chloroform extraction
- Ethanol precipitationEthanol precipitationEthanol precipitation is a method used to purify and/or concentrate RNA, DNA and polysaccharides such as pectin and xyloglucan from aqueous solutions.- Theory :DNA is polar due to its highly charged phosphate backbone...
- DNA separation by silica adsorptionDNA separation by silica adsorptionDNA Separation by Silica Adsorption is an important method of DNA separation that is used in novel technologies that use micro-channels. The principle behind this type of separation relies on DNA molecules binding to silica surfaces in the presence of certain salts and under certain pH...

