
Claisen condensation
Encyclopedia
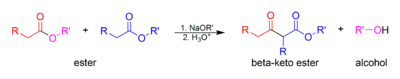
Condensation reaction
A condensation reaction is a chemical reaction in which two molecules or moieties combine to form one single molecule, together with the loss of a small molecule. When this small molecule is water, it is known as a dehydration reaction; other possible small molecules lost are hydrogen chloride,...
(not to be confused with the Claisen rearrangement
Claisen rearrangement
The Claisen rearrangement is a powerful carbon-carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen...
) is a carbon–carbon bond forming reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
that occurs between two ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s or one ester and another carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
. It is named after Rainer Ludwig Claisen
Rainer Ludwig Claisen
Rainer Ludwig Claisen was a famous German chemist best known for his work with condensations of carbonyls and sigmatropic rearrangements. He was born in Cologne as the son of a jurist and studied chemistry at the university of Bonn , where he became a member of K.St.V. Arminia...
, who first published his work on the reaction in 1881 .
Requirements
At least one of the reagents must be enolizable (have an α-protonAlpha carbon
The alpha carbon in organic chemistry refers to the first carbon that attaches to a functional group . By extension, the second carbon is the beta carbon, and so on....
and be able to undergo deprotonation
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
to form the enolate anion
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
). There are a number of different combinations of enolizable and nonenolizable carbonyl compounds that form a few different types of Claisen condensations.
The base used must not interfere with the reaction by undergoing nucleophilic
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
substitution
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
or addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
with a carbonyl carbon. For this reason, the conjugate sodium alkoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
base of the alcohol formed (e.g. sodium ethoxide
Sodium ethoxide
Sodium ethoxide is an alkoxide salt with the chemical formula C2H5ONa.-Preparation:It is commercially available as a white solid, or as a solution in ethanol. It is easily prepared in the laboratory by reacting sodium metal with ethanol:...
if ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
is formed) is often used, since the alkoxide is regenerated. In mixed Claisen condensations, a non-nucleophilic base such as lithium diisopropylamide
Lithium diisopropylamide
Lithium diisopropylamide is the chemical compound with the formula [2CH]2NLi. Generally abbreviated LDA, it is a strong base used in organic chemistry for the deprotonation of weakly acidic compounds. The reagent has been widely accepted because it is soluble in non-polar organic solvents and it...
, or LDA, may be used, since only one compound is enolizable. LDA is not commonly used in the classic Claisen or Dieckmann condensations due to enolization of the electrophilic ester.
The alkoxy portion of the ester must be a relatively good leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
. Methyl
Methyl group
Methyl group is a functional group derived from methane, containing one carbon atom bonded to three hydrogen atoms —CH3. The group is often abbreviated Me. Such hydrocarbon groups occur in many organic compounds. The methyl group can be found in three forms: anion, cation and radical. The anion...
and ethyl
Ethyl group
In chemistry, an ethyl group is an alkyl substituent derived from ethane . It has the formula -C2H5 and is very often abbreviated -Et.Ethylation is the formation of a compound by introduction of the ethyl functional group, C2H5....
esters, which yields methoxide and ethoxide, respectively, are commonly used.
Types
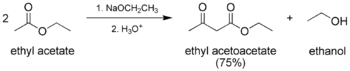
The classic Claisen condensation, where only one enolizable ester is used.
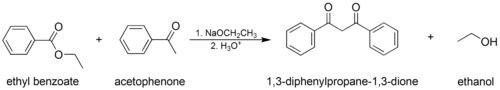
The mixed (or "crossed") Claisen condensation, where an enolizable ester or ketone and a nonenolizable ester are used.
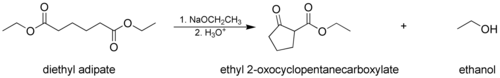
The Dieckmann condensation
Dieckmann condensation
The Dieckmann condensation is the intramolecular chemical reaction of diesters with base to give β-ketoesters. It is named after the German chemist Walter Dieckmann . The equivalent intermolecular reaction is the Claisen condensation....
, where a molecule with two ester groups reacts intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
ly, forming a cyclic
Cyclic compound
In chemistry, a cyclic compound is a compound in which a series of atoms is connected to form a loop or ring.While the vast majority of cyclic compounds are organic, a few inorganic substances form cyclic compounds as well, including sulfur, silanes, phosphanes, phosphoric acid, and triboric acid. ...
β-keto ester. In this case, the ring formed must not be strained
Ring strain
In organic chemistry, ring strain is the tendency of a cyclic molecule, such as cyclopropane, to destabilize when its atoms are in non-favorable high energy spatial orientations...
, usually a 5- or 6-membered chain or ring.
Mechanism
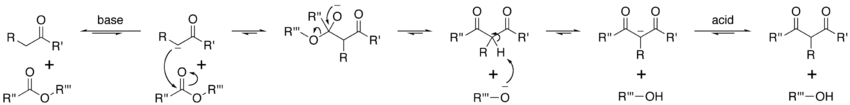
Delocalized electron
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or one covalent bond....
of electrons. Next, the carbonyl carbon of the (other) ester is nucleophilically attacked by the enolate anion. The alkoxy group is then eliminated (resulting in (re)generation of the alkoxide), and the alkoxide removes the newly-formed doubly α-proton to form a new, highly resonance-stabilized enolate anion. Aqueous acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
(e.g. sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
or phosphoric acid
Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
) is added in the final step to neutralize
Neutralization
In chemistry, neutralization, or neutralisation is a chemical reaction in which an acid and a base react to form a salt. Water is frequently, but not necessarily, produced as well. Neutralizations with Arrhenius acids and bases always produce water:Y and X represent a monovalent cation and anion...
the enolate and any base still present. The newly-formed β-keto ester or β-diketone is then isolated. Note that the reaction requires a stoichiometric
Stoichiometry
Stoichiometry is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of whole numbers...
amount of base as the removal of the doubly α-proton thermodynamically drives the otherwise endergonic
Endergonic
Endergonic means "absorbing energy in the form of work." Endergonic reactions are not spontaneous...
reaction. That is, Claisen condensation
Claisen condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone...
does not work with substrates
Substrate (chemistry)
In chemistry, a substrate is the chemical species being observed, which reacts with a reagent. This term is highly context-dependent. In particular, in biochemistry, an enzyme substrate is the material upon which an enzyme acts....
having only one α-hydrogen because of the driving force effect of deprotonation of the β-keto ester in the last step.
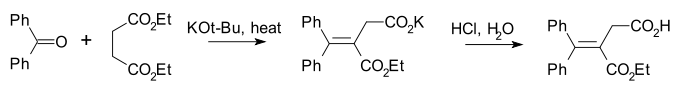
Stobbe condensation
The Stobbe condensation is a modification specific for the diethyl esterEster
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
of succinic acid
Succinic acid
Succinic acid is a dicarboxylic acid. Succinate plays a biochemical role in the citric acid cycle. The name derives from Latin succinum, meaning amber, from which the acid may be obtained....
requiring less strong bases . An example is its reaction with benzophenone
Benzophenone
Benzophenone is the organic compound with the formula 2CO, generally abbreviated Ph2CO. Benzophenone is a widely used building block in organic chemistry, being the parent diarylketone.-Uses:...
:
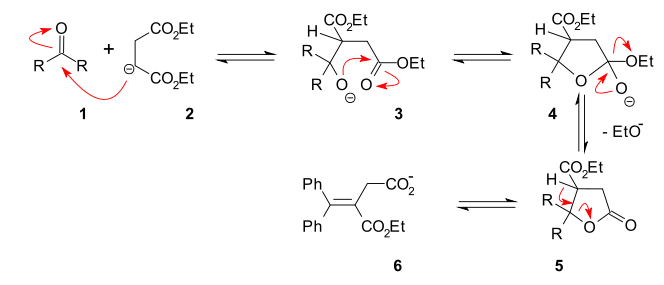
A reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
that explains the formation of both an ester group and a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
group is centered around a lactone
Lactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
intermediate (5):
See also
- Aldol condensationAldol condensationAn aldol condensation is an organic reaction in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by a dehydration to give a conjugated enone....
- fatty acid synthesis
- Polyketide synthasePolyketide synthasePolyketide synthases are a family of multi-domain enzymes or enzyme complexes that produce polyketides, a large class of secondary metabolites, in bacteria, fungi, plants, and a few animal lineages...
- Dieckmann condensationDieckmann condensationThe Dieckmann condensation is the intramolecular chemical reaction of diesters with base to give β-ketoesters. It is named after the German chemist Walter Dieckmann . The equivalent intermolecular reaction is the Claisen condensation....

