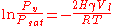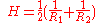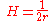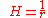Capillary condensation
Encyclopedia
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
from the vapor [phase] into a porous medium
Porous medium
A porous medium is a material containing pores . The skeletal portion of the material is often called the "matrix" or "frame". The pores are typically filled with a fluid...
proceeds to the point at which pore spaces become filled with condensed liquid from the vapor [phase]." The unique aspect of capillary condensation is that vapor condensation occurs below the saturation vapor pressure, Psat, of the pure liquid. This result is due to an increased number of van der Waals
Van der Waals force
In physical chemistry, the van der Waals force , named after Dutch scientist Johannes Diderik van der Waals, is the sum of the attractive or repulsive forces between molecules other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral...
interactions between vapor phase molecules inside the confined space of a capillary. Once condensation has occurred, a meniscus
Meniscus
The meniscus is the curve in the upper surface of a liquid close to the surface of the container or another object, caused by surface tension. It can be either convex or concave. A convex meniscus occurs when the molecules have a stronger attraction to each other than to the material of the...
immediately forms at the liquid-vapor interface which allows for equilibrium
Vapor-liquid equilibrium
Vapor–liquid equilibrium is a condition where a liquid and its vapor are in equilibrium with each other, a condition or state where the rate of evaporation equals the rate of condensation on a molecular level such that there is no net vapor-liquid interconversion...
below the saturation vapor pressure. Meniscus
Meniscus
The meniscus is the curve in the upper surface of a liquid close to the surface of the container or another object, caused by surface tension. It can be either convex or concave. A convex meniscus occurs when the molecules have a stronger attraction to each other than to the material of the...
formation is dependent on the surface tension
Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
of the liquid and the shape of the capillary, as shown by the Young-Laplace equation. As with any liquid-vapor interface involving a menisci, the Kelvin equation
Kelvin equation
The Kelvin equation describes the change in vapour pressure due to a curved liquid/vapor interface with radius r . The Kelvin equation is used for determination of pore size distribution of a porous medium using adsorption porosimetry...
provides a relation for the difference between the equilibrium vapor pressure and the saturation vapor pressure. A capillary does not necessarily have to be a tubular, closed shape, but can be any confined space with respect to its surroundings.
Capillary condensation is an important factor in both naturally occurring and synthetic porous structures. In these structures, scientists use the concept of capillary condensation to determine pore size distribution and surface area
Surface area
Surface area is the measure of how much exposed area a solid object has, expressed in square units. Mathematical description of the surface area is considerably more involved than the definition of arc length of a curve. For polyhedra the surface area is the sum of the areas of its faces...
though adsorption isotherms. Synthetic applications such as sintering
Sintering
Sintering is a method used to create objects from powders. It is based on atomic diffusion. Diffusion occurs in any material above absolute zero, but it occurs much faster at higher temperatures. In most sintering processes, the powdered material is held in a mold and then heated to a temperature...
of materials are also highly dependent on bridging effects resulting from capillary condensation. In contrast to the advantages of capillary condensation, it can also cause many problems in materials science applications such as Atomic Force Microscopy and Microelectromechanical Systems
Microelectromechanical systems
Microelectromechanical systems is the technology of very small mechanical devices driven by electricity; it merges at the nano-scale into nanoelectromechanical systems and nanotechnology...
.
Kelvin equation
The Kelvin equationKelvin equation
The Kelvin equation describes the change in vapour pressure due to a curved liquid/vapor interface with radius r . The Kelvin equation is used for determination of pore size distribution of a porous medium using adsorption porosimetry...
can be used to describe the phenomenon of capillary condensation due to the presence of a curved meniscus
Meniscus
The meniscus is the curve in the upper surface of a liquid close to the surface of the container or another object, caused by surface tension. It can be either convex or concave. A convex meniscus occurs when the molecules have a stronger attraction to each other than to the material of the...
.
Where...
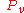 = equilibrium vapor pressure
= equilibrium vapor pressureVapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
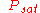 = saturation vapor pressure
= saturation vapor pressureVapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
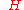 = mean curvature
= mean curvatureCurvature
In mathematics, curvature refers to any of a number of loosely related concepts in different areas of geometry. Intuitively, curvature is the amount by which a geometric object deviates from being flat, or straight in the case of a line, but this is defined in different ways depending on the context...
of meniscus
Meniscus
The meniscus is the curve in the upper surface of a liquid close to the surface of the container or another object, caused by surface tension. It can be either convex or concave. A convex meniscus occurs when the molecules have a stronger attraction to each other than to the material of the...
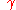 = liquid/vapor surface tension
= liquid/vapor surface tensionSurface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
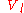 = liquid molar volume
= liquid molar volumeMolar volume
The molar volume, symbol Vm, is the volume occupied by one mole of a substance at a given temperature and pressure. It is equal to the molar mass divided by the mass density...
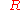 = ideal gas constant
= ideal gas constant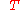 = temperature
= temperatureThis equation, shown above, governs all equilibrium systems involving meniscus
Meniscus
The meniscus is the curve in the upper surface of a liquid close to the surface of the container or another object, caused by surface tension. It can be either convex or concave. A convex meniscus occurs when the molecules have a stronger attraction to each other than to the material of the...
and provides mathematical reasoning for the fact that condensation of a given species occurs below the saturation vapor pressure
Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
(Pv < Psat) inside a capillary. At the heart of the Kelvin equation
Kelvin equation
The Kelvin equation describes the change in vapour pressure due to a curved liquid/vapor interface with radius r . The Kelvin equation is used for determination of pore size distribution of a porous medium using adsorption porosimetry...
is the pressure difference between the liquid and vapor phases, which comes as a contrast to traditional phase diagrams where phase equilibrium occurs at a single pressure, known as Psat, for a given temperature. This pressure drop (
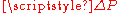 ) is due solely to the liquid/vapor surface tension
) is due solely to the liquid/vapor surface tensionSurface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
and curvature
Curvature
In mathematics, curvature refers to any of a number of loosely related concepts in different areas of geometry. Intuitively, curvature is the amount by which a geometric object deviates from being flat, or straight in the case of a line, but this is defined in different ways depending on the context...
of the meniscus
Meniscus
The meniscus is the curve in the upper surface of a liquid close to the surface of the container or another object, caused by surface tension. It can be either convex or concave. A convex meniscus occurs when the molecules have a stronger attraction to each other than to the material of the...
, as described in the Young-Laplace equation.
In the Kelvin equation
Kelvin equation
The Kelvin equation describes the change in vapour pressure due to a curved liquid/vapor interface with radius r . The Kelvin equation is used for determination of pore size distribution of a porous medium using adsorption porosimetry...
, the saturation vapor pressure
Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
, surface tension
Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
, and molar volume
Molar volume
The molar volume, symbol Vm, is the volume occupied by one mole of a substance at a given temperature and pressure. It is equal to the molar mass divided by the mass density...
are all inherent properties of the species at equilibrium and are considered constants with respect to the system. Temperature is also a constant in the Kelvin equation
Kelvin equation
The Kelvin equation describes the change in vapour pressure due to a curved liquid/vapor interface with radius r . The Kelvin equation is used for determination of pore size distribution of a porous medium using adsorption porosimetry...
as it is a function of the saturation vapor pressure
Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
and vice versa. Therefore, the variables that govern capillary condensation most are the equilibrium vapor pressure
Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
and the mean curvature
Curvature
In mathematics, curvature refers to any of a number of loosely related concepts in different areas of geometry. Intuitively, curvature is the amount by which a geometric object deviates from being flat, or straight in the case of a line, but this is defined in different ways depending on the context...
of the meniscus
Meniscus
The meniscus is the curve in the upper surface of a liquid close to the surface of the container or another object, caused by surface tension. It can be either convex or concave. A convex meniscus occurs when the molecules have a stronger attraction to each other than to the material of the...
.
Dependence of Pv/Psat
The relation of equilibrium vapor pressureVapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
to the saturation vapor pressure can be thought of as a relative humidity
Humidity
Humidity is a term for the amount of water vapor in the air, and can refer to any one of several measurements of humidity. Formally, humid air is not "moist air" but a mixture of water vapor and other constituents of air, and humidity is defined in terms of the water content of this mixture,...
measurement for the atmosphere. As Pv/Psat increases, vapor will continue to condense inside a given capillary. If Pv/Psat decreases, liquid will begin to evaporate into the atmosphere as vapor molecules. The figure below demonstrates four different systems in which Pv/Psat is increasing from left to right.
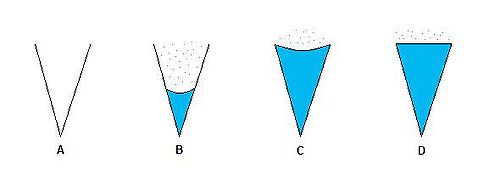
System B → Pv=P1
Vapor-liquid equilibrium
Vapor–liquid equilibrium is a condition where a liquid and its vapor are in equilibrium with each other, a condition or state where the rate of evaporation equals the rate of condensation on a molecular level such that there is no net vapor-liquid interconversion...
is reached
System C → Pv=P2
Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
is increased condensation
Condensation
Condensation is the change of the physical state of matter from gaseous phase into liquid phase, and is the reverse of vaporization. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition....
continues in order to satisfy the Kelvin equation
Kelvin equation
The Kelvin equation describes the change in vapour pressure due to a curved liquid/vapor interface with radius r . The Kelvin equation is used for determination of pore size distribution of a porous medium using adsorption porosimetry...
System D → Pv=Pmax
Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
is increased to its maximum allowed value and the pore is filled completely
This figure is used to demonstrate the concept that by increasing the vapor pressure
Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
in a given system, more condensation will occur. In a porous medium
Porous medium
A porous medium is a material containing pores . The skeletal portion of the material is often called the "matrix" or "frame". The pores are typically filled with a fluid...
, capillary condensation will always occur if Pv≠0.
Dependence on curvature
The Kelvin equationKelvin equation
The Kelvin equation describes the change in vapour pressure due to a curved liquid/vapor interface with radius r . The Kelvin equation is used for determination of pore size distribution of a porous medium using adsorption porosimetry...
indicates that as Pv/Psat increases inside a capillary, the radius of curvature
Radius of curvature (mathematics)
In geometry, the radius of curvature, R, of a curve at a point is a measure of the radius of the circular arc which best approximates the curve at that point. If this value taken to be positive when the curve turns anticlockwise and negative when the curve turns clockwise...
will also increase, creating a flatter interface. (Note: This is not to say that larger radii of curvature result in more vapor condensation. See the discussion on contact angle below.) Figure 2 above demonstrates this dependence in a simple situation whereby the capillary radius is expanding toward the opening of the capillary and thus vapor condensation occurs smoothly over a range of vapor pressures. In a parallel situation, where the capillary radius is constant throughout its height, vapor condensation would occur much more rapidly, reaching the equilibrium radius of curvature (Kelvin radius) as quickly as possible. This dependence on pore geometry and curvature
Curvature
In mathematics, curvature refers to any of a number of loosely related concepts in different areas of geometry. Intuitively, curvature is the amount by which a geometric object deviates from being flat, or straight in the case of a line, but this is defined in different ways depending on the context...
can result in hysteresis
Hysteresis
Hysteresis is the dependence of a system not just on its current environment but also on its past. This dependence arises because the system can be in more than one internal state. To predict its future evolution, either its internal state or its history must be known. If a given input alternately...
and vastly different liquid/vapor equilibria over very small ranges in pressure.
It is also worthy to mention that different pore geometries result in different types of curvature
Curvature
In mathematics, curvature refers to any of a number of loosely related concepts in different areas of geometry. Intuitively, curvature is the amount by which a geometric object deviates from being flat, or straight in the case of a line, but this is defined in different ways depending on the context...
. In scientific studies of capillary condensation, the hemispherical meniscus
Meniscus
The meniscus is the curve in the upper surface of a liquid close to the surface of the container or another object, caused by surface tension. It can be either convex or concave. A convex meniscus occurs when the molecules have a stronger attraction to each other than to the material of the...
situation (that resulting from a perfectly cylindrical pore) is most often investigated due to its simplicity. Cylindrical menisci are also useful systems because they typically result from scratches, cuts, and slit-type capillaries in surfaces. Many other types of curvature
Curvature
In mathematics, curvature refers to any of a number of loosely related concepts in different areas of geometry. Intuitively, curvature is the amount by which a geometric object deviates from being flat, or straight in the case of a line, but this is defined in different ways depending on the context...
are possible and equations for the curvature
Curvature
In mathematics, curvature refers to any of a number of loosely related concepts in different areas of geometry. Intuitively, curvature is the amount by which a geometric object deviates from being flat, or straight in the case of a line, but this is defined in different ways depending on the context...
of menisci are readily available at numerous sources. Those for the hemispherical and cylindrical menisci are shown below.
General Curvature Equation:
Cylinder:
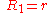
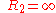
Hemisphere:
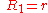
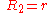
Dependence on contact angle
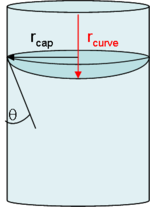
Contact angle
The contact angle is the angle at which a liquid/vapor interface meets a solid surface. The contact angle is specific for any given system and is determined by the interactions across the three interfaces. Most often the concept is illustrated with a small liquid droplet resting on a flat...
, or wetting angle, is a very important parameter in real systems where perfect wetting
Wetting
Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. The degree of wetting is determined by a force balance between adhesive and cohesive forces.Wetting is important in the bonding or adherence of...
(
 = 0o) is hardly ever achieved. The Young Equation provides reasoning for contact angle
= 0o) is hardly ever achieved. The Young Equation provides reasoning for contact angleContact angle
The contact angle is the angle at which a liquid/vapor interface meets a solid surface. The contact angle is specific for any given system and is determined by the interactions across the three interfaces. Most often the concept is illustrated with a small liquid droplet resting on a flat...
involvement in capillary condensation. The Young Equation explains that the surface tension
Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
between the liquid and vapor phases is scaled to the cosine of the contact angle
Contact angle
The contact angle is the angle at which a liquid/vapor interface meets a solid surface. The contact angle is specific for any given system and is determined by the interactions across the three interfaces. Most often the concept is illustrated with a small liquid droplet resting on a flat...
. As shown in the figure to the right, the contact angle
Contact angle
The contact angle is the angle at which a liquid/vapor interface meets a solid surface. The contact angle is specific for any given system and is determined by the interactions across the three interfaces. Most often the concept is illustrated with a small liquid droplet resting on a flat...
between a condensed liquid and the inner wall of a capillary can affect the radius of curvature a great deal. For this reason, contact angle
Contact angle
The contact angle is the angle at which a liquid/vapor interface meets a solid surface. The contact angle is specific for any given system and is determined by the interactions across the three interfaces. Most often the concept is illustrated with a small liquid droplet resting on a flat...
is coupled inherently to the curvature term of the Kelvin equation
Kelvin equation
The Kelvin equation describes the change in vapour pressure due to a curved liquid/vapor interface with radius r . The Kelvin equation is used for determination of pore size distribution of a porous medium using adsorption porosimetry...
. As the contact angle
Contact angle
The contact angle is the angle at which a liquid/vapor interface meets a solid surface. The contact angle is specific for any given system and is determined by the interactions across the three interfaces. Most often the concept is illustrated with a small liquid droplet resting on a flat...
increases, the radius of curvature
Radius of curvature (mathematics)
In geometry, the radius of curvature, R, of a curve at a point is a measure of the radius of the circular arc which best approximates the curve at that point. If this value taken to be positive when the curve turns anticlockwise and negative when the curve turns clockwise...
will increase as well. This is to say that a system with perfect wetting
Wetting
Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. The degree of wetting is determined by a force balance between adhesive and cohesive forces.Wetting is important in the bonding or adherence of...
will exhibit a larger amount of liquid in its pores than a system with non-perfect wetting
Wetting
Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. The degree of wetting is determined by a force balance between adhesive and cohesive forces.Wetting is important in the bonding or adherence of...
(
 > 0o). Also, in systems where
> 0o). Also, in systems where  = 0o the radius of curvature is equal to the capillary radius. Due to these complications caused by contact angle
= 0o the radius of curvature is equal to the capillary radius. Due to these complications caused by contact angleContact angle
The contact angle is the angle at which a liquid/vapor interface meets a solid surface. The contact angle is specific for any given system and is determined by the interactions across the three interfaces. Most often the concept is illustrated with a small liquid droplet resting on a flat...
, scientific studies are often designed to assume
 = 0o.
= 0o.Odd pore geometries
In both naturally occurring and synthetic porous structures, the geometry of pores and capillaries is almost never perfectly cylindrical. Often, porous media contain networks of capillaries, much like a sponge. Since pore geometry affects the shape and curvature of an equilibrium meniscus, the Kelvin equation could be represented differently every time the meniscus changes along a “snake-like” capillary. This makes the analysis via the Kelvin equation complicated very quickly. Adsorption isotherm studies utilizing capillary condensation are still the main method for determining pore size and shape. With advancements in synthetic techniques and instrumentation, very well ordered porous structures are now available which circumvent the problem of odd-pore geometries in engineered systems.Hysteresis
Non-uniform pore geometries often lead to differences in adsorption and desorption pathways within a capillary. This deviation in the two is called a hysteresis and is characteristic of many path dependent processes. For example, if a capillary’s radius increases sharply, then capillary condensation (adsorption) will cease until an equilibrium vapor pressure is reached which satisfies the larger pore radius. However, during evaporation (desorption), liquid will remain filled to the larger pore radius until an equilibrium vapor pressure that satisfies the smaller pore radius is reached. The resulting plot of adsorbed volume versus relative humidity yields a hysteresis “loop.” This loop is seen in all hysteresis governed processes and gives direct meaning the term “path dependent.” The concept of hysteresis was explained indirectly in the curvature section of this article; however, here we are speaking in terms of a single capillary instead of a distribution of random pore sizes.Hysteresis in capillary condensation has been shown to be minimized at higher temperatures.
Accounting for small capillary radii
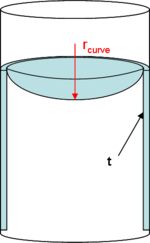
Bridging effects
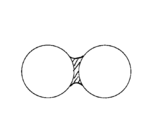
Sintering
Sintering is a method used to create objects from powders. It is based on atomic diffusion. Diffusion occurs in any material above absolute zero, but it occurs much faster at higher temperatures. In most sintering processes, the powdered material is held in a mold and then heated to a temperature...
; bridging implies the formation of a meniscus that brings two surfaces or particles in contact with each other without direct integration or loss of individuality.
Atomic force microscopy
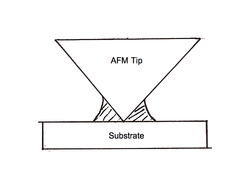
Meniscus
The meniscus is the curve in the upper surface of a liquid close to the surface of the container or another object, caused by surface tension. It can be either convex or concave. A convex meniscus occurs when the molecules have a stronger attraction to each other than to the material of the...
, as is stated above. While this is sometimes a desirable effect, in the case of Atomic Force Microscopy (AFM) this is an exceedingly undesirable consequence. AFM is generally operated in one of two main modes, contact mode and non-contact mode. While studies have been done on the formation of the meniscus
Meniscus
The meniscus is the curve in the upper surface of a liquid close to the surface of the container or another object, caused by surface tension. It can be either convex or concave. A convex meniscus occurs when the molecules have a stronger attraction to each other than to the material of the...
between the tip and the sample, no concrete conclusion can be drawn as to the optimum height away from the sample the tip can be without this side effect occurring. Scientific studies have been done on the relation between relative humidity and the height of the meniscus
Meniscus
The meniscus is the curve in the upper surface of a liquid close to the surface of the container or another object, caused by surface tension. It can be either convex or concave. A convex meniscus occurs when the molecules have a stronger attraction to each other than to the material of the...
created by capillary condensation. One particular study, done by Weeks, illustrated that with the increase in relative humidity, there is a large increase in the size of the meniscus
Meniscus
The meniscus is the curve in the upper surface of a liquid close to the surface of the container or another object, caused by surface tension. It can be either convex or concave. A convex meniscus occurs when the molecules have a stronger attraction to each other than to the material of the...
. This study also states that no meniscus
Meniscus
The meniscus is the curve in the upper surface of a liquid close to the surface of the container or another object, caused by surface tension. It can be either convex or concave. A convex meniscus occurs when the molecules have a stronger attraction to each other than to the material of the...
formation is observed when the relative humidity is less than 70%, although there is uncertainty in this conclusion due to limits of resolution. The meniscus
Meniscus
The meniscus is the curve in the upper surface of a liquid close to the surface of the container or another object, caused by surface tension. It can be either convex or concave. A convex meniscus occurs when the molecules have a stronger attraction to each other than to the material of the...
effect with AFM is rather detrimental because of the hard time that AFM already has with accurate information in the x and y planes of the sample. Meniscus
Meniscus
The meniscus is the curve in the upper surface of a liquid close to the surface of the container or another object, caused by surface tension. It can be either convex or concave. A convex meniscus occurs when the molecules have a stronger attraction to each other than to the material of the...
formation causes the tip to have an elongated geometry which in turn distorts the already semi-inaccurate information gathered from the x and y directions.
Although undesirable for imaging purposes, the formation of the meniscus is the basis of the Dip-Pen Nanolithography technique.
Sintering
Sintering
Sintering is a method used to create objects from powders. It is based on atomic diffusion. Diffusion occurs in any material above absolute zero, but it occurs much faster at higher temperatures. In most sintering processes, the powdered material is held in a mold and then heated to a temperature...
is a common practice used widely with both metals and ceramic
Ceramic
A ceramic is an inorganic, nonmetallic solid prepared by the action of heat and subsequent cooling. Ceramic materials may have a crystalline or partly crystalline structure, or may be amorphous...
materials. Sintering
Sintering
Sintering is a method used to create objects from powders. It is based on atomic diffusion. Diffusion occurs in any material above absolute zero, but it occurs much faster at higher temperatures. In most sintering processes, the powdered material is held in a mold and then heated to a temperature...
is a direct application of capillary condensation, because of the adhesion effects of dust and powders. This application can be seen directly in sol-gel thin film synthesis. The sol-gel is a colloid
Colloid
A colloid is a substance microscopically dispersed evenly throughout another substance.A colloidal system consists of two separate phases: a dispersed phase and a continuous phase . A colloidal system may be solid, liquid, or gaseous.Many familiar substances are colloids, as shown in the chart below...
solution which is placed on a substrate, usually through a dip-coating method. After being placed onto the substrate, a source of heat is applied to evaporate all undesired liquid. While the liquid is evaporating, the particles that were once in solution adhere to each other, thus forming a thin film.
MEMS
Microelectromechanical systemsMicroelectromechanical systems
Microelectromechanical systems is the technology of very small mechanical devices driven by electricity; it merges at the nano-scale into nanoelectromechanical systems and nanotechnology...
(MEMS) are used in a number of different applications and have become increasingly more prevalent in nanoscale applications. However, due to their small size they run into problems with stiction
Stiction
Stiction is the static friction that needs to be overcome to enable relative motion of stationary objects in contact. The term is a portmanteau of the term "static friction", perhaps also influenced by the verb "stick"....
, caused by capillary condensation among other forces. Intense research in the area of Microelectromechanical systems
Microelectromechanical systems
Microelectromechanical systems is the technology of very small mechanical devices driven by electricity; it merges at the nano-scale into nanoelectromechanical systems and nanotechnology...
has been focused on finding ways to reduce stiction
Stiction
Stiction is the static friction that needs to be overcome to enable relative motion of stationary objects in contact. The term is a portmanteau of the term "static friction", perhaps also influenced by the verb "stick"....
in the fabrication of Microelectromechanical systems
Microelectromechanical systems
Microelectromechanical systems is the technology of very small mechanical devices driven by electricity; it merges at the nano-scale into nanoelectromechanical systems and nanotechnology...
and when they are being used. Srinivasan et al. did a study in 1998 looking at applying different types of Self-assembled monolayers (SAMs) to the surfaces of Microelectromechanical systems
Microelectromechanical systems
Microelectromechanical systems is the technology of very small mechanical devices driven by electricity; it merges at the nano-scale into nanoelectromechanical systems and nanotechnology...
in hopes of reducing stiction
Stiction
Stiction is the static friction that needs to be overcome to enable relative motion of stationary objects in contact. The term is a portmanteau of the term "static friction", perhaps also influenced by the verb "stick"....
or getting rid of it altogether. They found that using OTS (octadecyltrichlorosilane) coatings reduced both types of stiction
Stiction
Stiction is the static friction that needs to be overcome to enable relative motion of stationary objects in contact. The term is a portmanteau of the term "static friction", perhaps also influenced by the verb "stick"....
.
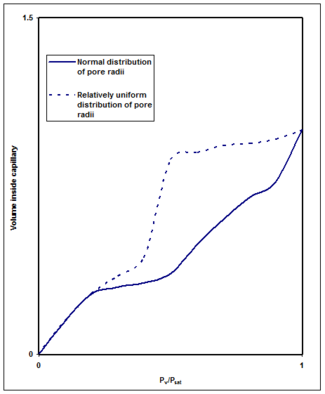
Pore size distribution
Pores that are not of the same size will fill at different values of pressure, with the smaller ones filling first. This difference in filling rate can be a beneficial application of capillary condensation. Many materials have different pore sizes with ceramics being one of the most commonly encountered. In materials with different pore sizes, curves can be constructed similar to Figure 7. A detailed analysis of the shape of these isotherms is done using the Kelvin equationKelvin equation
The Kelvin equation describes the change in vapour pressure due to a curved liquid/vapor interface with radius r . The Kelvin equation is used for determination of pore size distribution of a porous medium using adsorption porosimetry...
. This enables the pore size distribution to be determined. While this is a relatively simple method of analyzing the isotherms, a more in depth analysis of the isotherms is done using the BET
BET theory
BET theory aims to explain the physical adsorption of gas molecules on a solid surface and serves as the basis for an important analysis technique for the measurement of the specific surface area of a material...
method. Another method of determining the pore size distribution is by using a procedure known as Mercury Injection Porosimetry. This uses the volume of mercury taken up by the solid as the pressure increases to create the same isotherms mentioned above. An application where pore size is beneficial is in regards to oil recovery. When recovering oil from tiny pores, it is useful to inject gas and water into the pore. The gas will then occupy the space where the oil once was, mobilizing the oil, and then the water will displace some of the oil forcing it to leave the pore.
See also
- AdsorptionAdsorptionAdsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
- Atomic Force Microscopy
- BET theoryBET theoryBET theory aims to explain the physical adsorption of gas molecules on a solid surface and serves as the basis for an important analysis technique for the measurement of the specific surface area of a material...
- Capillarity
- CurvatureCurvatureIn mathematics, curvature refers to any of a number of loosely related concepts in different areas of geometry. Intuitively, curvature is the amount by which a geometric object deviates from being flat, or straight in the case of a line, but this is defined in different ways depending on the context...
- Disjoining pressureDisjoining pressureDisjoining pressure , in surface chemistry, according to an IUPAC definition, arises from an attractive interaction between two surfaces...
- Kelvin equationKelvin equationThe Kelvin equation describes the change in vapour pressure due to a curved liquid/vapor interface with radius r . The Kelvin equation is used for determination of pore size distribution of a porous medium using adsorption porosimetry...
- Self-assembled monolayers
- SinteringSinteringSintering is a method used to create objects from powders. It is based on atomic diffusion. Diffusion occurs in any material above absolute zero, but it occurs much faster at higher temperatures. In most sintering processes, the powdered material is held in a mold and then heated to a temperature...
- Microelectromechanical systemsMicroelectromechanical systemsMicroelectromechanical systems is the technology of very small mechanical devices driven by electricity; it merges at the nano-scale into nanoelectromechanical systems and nanotechnology...
- MeniscusMeniscusThe meniscus is the curve in the upper surface of a liquid close to the surface of the container or another object, caused by surface tension. It can be either convex or concave. A convex meniscus occurs when the molecules have a stronger attraction to each other than to the material of the...
- Sol-gel
- ColloidColloidA colloid is a substance microscopically dispersed evenly throughout another substance.A colloidal system consists of two separate phases: a dispersed phase and a continuous phase . A colloidal system may be solid, liquid, or gaseous.Many familiar substances are colloids, as shown in the chart below...
- CeramicCeramicA ceramic is an inorganic, nonmetallic solid prepared by the action of heat and subsequent cooling. Ceramic materials may have a crystalline or partly crystalline structure, or may be amorphous...