Biliverdin reductase
Encyclopedia
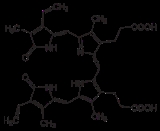
Biliverdin reductase (BVR) is an enzyme
found in the liver that facilitates the conversion of biliverdin
to bilirubin
. It accomplishes this through the reduction
of a double-bond between the second and third pyrrole
ring into a single-bond.
There are two isozyme
s, in humans, each encoded by its own gene, biliverdin reductase A (BLVRA) and biliverdin reductase B (BLVRB).
by reducing its double-bond between the pyrrole rings into a single-bond. It accomplishes this using NADPH + H+ as an electron donor, forming bilirubin
and NADP+ as products.
BVR catalyzes this reaction through an overlapping binding site including Lys18, Lys22, Lys179, Arg183, and Arg185 as key residues. This binding site attaches to biliverdin, and causes its dissociation from heme oxygenase (HO) (which catalyzes reaction of ferric heme --> biliverdin
), causing the subsequent reduction to bilirubin.
. BVR has also been determined to be a zinc-binding protein with each enzyme protein having one strong-binding zinc atom.
The C-terminal half of BVR contains the catalytic domain, which adopts a structure
containing a six-stranded beta-sheet that is flanked on one face by several alpha-helices
. This domain contains the catalytic
active site
, which reduces the gamma-methene bridge of the open tetrapyrrole, biliverdin IX alpha, to bilirubin
with the concomitant oxidation
of a NADH or NADPH cofactor
.
(ROS). This cycle allows for the neutralization of ROS, and the reuse of biliverdin products. Biliverdin also is replenished in the cycle with its formation from heme units through heme oxygenase
(HO) localized from the endoplasmic reticulum.
Bilirubin, being one of the last products of heme
degradation in the liver, is further processed and excreted in bile after conjugation with glucuronic acid
. In this way, BVR is essential in many mammals for the disposal of heme catabolites – especially in the fetus where the placental membranes are bilirubin-permeable but not biliverdin-permeable - aiding in the removal of potentially toxic protein build-up.
BVR has also more recently been recognized as a regulator of glucose metabolism and in cell growth and apoptosis control, due to its dual-specificity kinase character. This control over glucose metabolism indicates that BVR may play a role in pathogenesis of multiple metabolic diseases - the notable one being diabetes, by control of the upstream activator of insulin growth factor-1 (IGF-1) and mitogen-activated protein kinase (MAPK) signaling pathway.
and other types of oxidative stress-mediated diseases. The mechanism is due to the amplification of the potent antioxidant actions of bilirubin, as this can ameliorate free radical-mediated diseases.
Studies have shown that the BVR redox cycle is essential in providing physiological cytoprotection. Genetic knock-outs and reduced BVR levels have demonstrated increased formation of ROS, and results in augmented cell death. Cells that experienced a 90% reduction in BVR experienced three times normal ROS levels. Through this protective and amplifying cycle, BVR allows low concentrations of bilirubin to overcome 10,000-fold higher concentrations of ROS.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
found in the liver that facilitates the conversion of biliverdin
Biliverdin
Biliverdin is a green tetrapyrrolic bile pigment, and is a product of heme catabolism. It is the pigment responsible for a greenish color sometimes seen in bruises.- Metabolism :...
to bilirubin
Bilirubin
Bilirubin is the yellow breakdown product of normal heme catabolism. Heme is found in hemoglobin, a principal component of red blood cells. Bilirubin is excreted in bile and urine, and elevated levels may indicate certain diseases...
. It accomplishes this through the reduction
Organic redox reaction
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions because many reactions carry the name but do not actually involve electron...
of a double-bond between the second and third pyrrole
Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colourless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3...
ring into a single-bond.
There are two isozyme
Isozyme
Isozymes are enzymes that differ in amino acid sequence but catalyze the same chemical reaction. These enzymes usually display different kinetic parameters Isozymes (also known as isoenzymes) are enzymes that differ in amino acid sequence but catalyze the same chemical reaction. These enzymes...
s, in humans, each encoded by its own gene, biliverdin reductase A (BLVRA) and biliverdin reductase B (BLVRB).
Mechanism of catalysis
BVR acts on biliverdinBiliverdin
Biliverdin is a green tetrapyrrolic bile pigment, and is a product of heme catabolism. It is the pigment responsible for a greenish color sometimes seen in bruises.- Metabolism :...
by reducing its double-bond between the pyrrole rings into a single-bond. It accomplishes this using NADPH + H+ as an electron donor, forming bilirubin
Bilirubin
Bilirubin is the yellow breakdown product of normal heme catabolism. Heme is found in hemoglobin, a principal component of red blood cells. Bilirubin is excreted in bile and urine, and elevated levels may indicate certain diseases...
and NADP+ as products.
BVR catalyzes this reaction through an overlapping binding site including Lys18, Lys22, Lys179, Arg183, and Arg185 as key residues. This binding site attaches to biliverdin, and causes its dissociation from heme oxygenase (HO) (which catalyzes reaction of ferric heme --> biliverdin
Biliverdin
Biliverdin is a green tetrapyrrolic bile pigment, and is a product of heme catabolism. It is the pigment responsible for a greenish color sometimes seen in bruises.- Metabolism :...
), causing the subsequent reduction to bilirubin.
Structure
BVR is composed of two closely packed domains, between 247-415 amino acids long and containing a Rossmann foldRossmann fold
The Rossmann fold is a protein structural motif found in proteins that bind nucleotides, especially the cofactor NAD. The structure with two repeats is composed of six parallel beta strands linked to two pairs of alpha helices in the topological order beta-alpha-beta-alpha-beta...
. BVR has also been determined to be a zinc-binding protein with each enzyme protein having one strong-binding zinc atom.
The C-terminal half of BVR contains the catalytic domain, which adopts a structure
Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
containing a six-stranded beta-sheet that is flanked on one face by several alpha-helices
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
. This domain contains the catalytic
Biocatalysis
Biocatalysis is the use of natural catalysts, such as protein enzymes, to perform chemical transformations on organic compounds. Both enzymes that have been more or less isolated and enzymes still residing inside living cells are employed for this task....
active site
Active site
In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that...
, which reduces the gamma-methene bridge of the open tetrapyrrole, biliverdin IX alpha, to bilirubin
Bilirubin
Bilirubin is the yellow breakdown product of normal heme catabolism. Heme is found in hemoglobin, a principal component of red blood cells. Bilirubin is excreted in bile and urine, and elevated levels may indicate certain diseases...
with the concomitant oxidation
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
of a NADH or NADPH cofactor
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
.
Function
BVR works with the biliverdin/bilirubin redox cycle. It converts biliverdin to bilirubin (a strong antioxidant), which is then converted back into biliverdin through the actions of reactive oxygen speciesReactive oxygen species
Reactive oxygen species are chemically reactive molecules containing oxygen. Examples include oxygen ions and peroxides. Reactive oxygen species are highly reactive due to the presence of unpaired valence shell electrons....
(ROS). This cycle allows for the neutralization of ROS, and the reuse of biliverdin products. Biliverdin also is replenished in the cycle with its formation from heme units through heme oxygenase
Heme oxygenase
This reaction can occur in virtually every cell; the classic example is the formation of a bruise, which goes through different colors as it gradually heals: red heme to green biliverdin to yellow bilirubin...
(HO) localized from the endoplasmic reticulum.
Bilirubin, being one of the last products of heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
degradation in the liver, is further processed and excreted in bile after conjugation with glucuronic acid
Glucuronic acid
Glucuronic acid is a carboxylic acid. Its structure is similar to that of glucose. However, glucuronic acid's sixth carbon is oxidized to a carboxylic acid...
. In this way, BVR is essential in many mammals for the disposal of heme catabolites – especially in the fetus where the placental membranes are bilirubin-permeable but not biliverdin-permeable - aiding in the removal of potentially toxic protein build-up.
BVR has also more recently been recognized as a regulator of glucose metabolism and in cell growth and apoptosis control, due to its dual-specificity kinase character. This control over glucose metabolism indicates that BVR may play a role in pathogenesis of multiple metabolic diseases - the notable one being diabetes, by control of the upstream activator of insulin growth factor-1 (IGF-1) and mitogen-activated protein kinase (MAPK) signaling pathway.
Disease relevance
BVR acts as a means to regenerate bilirubin in a repeating redox cycle without significantly modifying the concentration of available bilirubin. With these levels maintained, it appears that BVR represents a new strategy for the treatment of multiple sclerosisMultiple sclerosis
Multiple sclerosis is an inflammatory disease in which the fatty myelin sheaths around the axons of the brain and spinal cord are damaged, leading to demyelination and scarring as well as a broad spectrum of signs and symptoms...
and other types of oxidative stress-mediated diseases. The mechanism is due to the amplification of the potent antioxidant actions of bilirubin, as this can ameliorate free radical-mediated diseases.
Studies have shown that the BVR redox cycle is essential in providing physiological cytoprotection. Genetic knock-outs and reduced BVR levels have demonstrated increased formation of ROS, and results in augmented cell death. Cells that experienced a 90% reduction in BVR experienced three times normal ROS levels. Through this protective and amplifying cycle, BVR allows low concentrations of bilirubin to overcome 10,000-fold higher concentrations of ROS.

