
Xanthate
Encyclopedia
Ancient Greek
Ancient Greek is the stage of the Greek language in the periods spanning the times c. 9th–6th centuries BC, , c. 5th–4th centuries BC , and the c. 3rd century BC – 6th century AD of ancient Greece and the ancient world; being predated in the 2nd millennium BC by Mycenaean Greek...
ξανθός ksantʰós, meaning “yellowish, golden”, and indeed most xanthate salts are yellow. These organosulfur compounds are important in two areas, the production of cellophane
Cellophane
Cellophane is a thin, transparent sheet made of regenerated cellulose. Its low permeability to air, oils, greases, bacteria and water makes it useful for food packaging...
and related polymers from cellulose
Cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to over ten thousand β linked D-glucose units....
and secondly in mining for the extraction of certain ores. They are also versatile intermediates in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. Xanthates also refer to ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
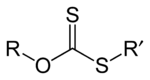
s of xanthic acid. These esters have the structure ROC(=S)SR'.
Formation and structure
Xanthate salts are produced by the reaction of an alcoholAlcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
with sodium or potassium hydroxide
Potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
and carbon disulfide
Carbon disulfide
Carbon disulfide is a colorless volatile liquid with the formula CS2. The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solvent...
:
- ROH + CS2 + KOH → ROCS2K + H2O
The reaction involves the attack of the alkoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
salt. For example, sodium ethoxide
Sodium ethoxide
Sodium ethoxide is an alkoxide salt with the chemical formula C2H5ONa.-Preparation:It is commercially available as a white solid, or as a solution in ethanol. It is easily prepared in the laboratory by reacting sodium metal with ethanol:...
gives sodium ethyl xanthate
Sodium ethyl xanthate
Sodium ethyl xanthate is an organosulfur compound with the chemical formula CH3CH2OCS2Na. It is a pale yellow powder, which characteristically hydrolyzes to release malodorous products...
. Virtually any alcohol can be used in this reaction. Technical grade xanthate salts are usually of 90–95% purity. Impurities include alkali-metal sulfide, sulfate, trithiocarbonates, thiosulfate
Thiosulfate
Thiosulfate is an oxyanion of sulfur. The prefix thio indicates that thiosulfate ion is a sulfate ion with one oxygen replaced by a sulfur. Thiosulfate occurs naturally and is produced by certain biochemical processes...
, sulfite, or carbonate as well as residual raw material such as alcohol and alkali hydroxide
Alkali hydroxide
The alkali hydroxides are a class of chemical compounds which are composed of an alkali metal cation and the hydroxide anion . The alkali hydroxides are:*Lithium hydroxide *Sodium hydroxide *Potassium hydroxide...
. These salts are available commercially as powder, granules, flakes, sticks, and solutions are available. China is a major exporter of granules.
Some commercially important xanthate salts include:
- sodium ethyl xanthateSodium ethyl xanthateSodium ethyl xanthate is an organosulfur compound with the chemical formula CH3CH2OCS2Na. It is a pale yellow powder, which characteristically hydrolyzes to release malodorous products...
(SEX), CH3CH2OCS2Na, - potassium ethyl xanthatePotassium ethyl xanthatePotassium ethyl xanthate is an organosulfur compound with the chemical formula CH3CH2OCS2K. It is a pale yellow powder that is used in the mining industry for the separation of ores. Unlike the related sodium ethyl xanthate, the potassium salt exists as an anhydrous salt.-Production and...
, CH3CH2OCS2K, - sodium isopropyl xanthate (SIPX)
- sodium isobutyl xanthate (SIBX)
- potassium amyl xanthate (PAX)
The OCS2 core of xanthate salts and esters is characteristically planar. The central carbon is sp2-hybridized.
Reactions
Xanthate salts characteristically decompose in acid:- ROCS2K + HCl → ROH + CS2 + KCl
This reaction is the reverse of the method for the preparation of the xanthate salts. The intermediate in the decomposition is the xanthic acid, ROC(S)SH, which can be isolated in certain cases.
Xanthate anions also undergo alkylation
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
to give xanthate esters, which are generally stable:
- ROCS2K + R'X → ROC(S)SR' + KCl
The C-O bond in these compounds are suscetpible to cleavage by the Barton–McCombie deoxygenation, which provides a means for deoxygenation of alcohols.
Analogous to their S-alkylation, xanthates bind to transition metal cations as bidentate ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s. The neutral complexes are soluble in organic solvents.
They can be oxidized to the so-called dixanthogens:
- 2 ROCS2Na + Cl2 → ROC(S)S2C(S)OR + 2 NaCl
Industrial applications
Cellulose reacts with carbon disulfideCarbon disulfide
Carbon disulfide is a colorless volatile liquid with the formula CS2. The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solvent...
(CS2) in presence of sodium hydroxide (NaOH) to produces sodium cellulose xanthate, which upon neutralization with sulfuric acid (H2SO4) gives viscose
Viscose
Viscose is a viscous organic liquid used to make rayon and cellophane. Viscose is becoming synonymous with rayon, a soft material commonly used in shirts, shorts, coats, jackets, and other outer wear.-Manufacture:...
rayon or cellophane
Cellophane
Cellophane is a thin, transparent sheet made of regenerated cellulose. Its low permeability to air, oils, greases, bacteria and water makes it useful for food packaging...
paper (Sellotape
Sellotape
Sellotape is a British brand of transparent, cellulose-based, pressure sensitive adhesive tape, and is the leading brand of clear, pressure sensitive tape in the United Kingdom. Sellotape is generally used for joining, sealing, attaching and mending...
or Scotch Tape
Scotch Tape
Scotch Tape is a brand name used for certain pressure sensitive tapes manufactured by 3M as part of the company's Scotch brand.- History :The precursor to the current tapes was developed in the 1930s in Minneapolis, Minnesota by Richard Drew to seal a then-new transparent material known as...
).
Xanthate salts are used as flotation
Flotation process
Flotation process is a method of separation widely used in the wastewater treatment and mineral processing industries.Various flotation processes include the following:* Dissolved air flotation...
agents in mineral processing. They are intermediates in the Chugaev elimination
Chugaev elimination
The Chugaev elimination is a chemical reaction that involves the elimination of water from alcohols to produce alkenes. The intermediate is a xanthate. It is named for its discoverer, the Russian chemist Lev Aleksandrovich Chugaev....
process and are used to control radical polymerisation
Living polymerization
In polymer chemistry, living polymerization is a form of addition polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is...
under the RAFT
RAFT (chemistry)
Reversible Addition-Fragmentation chain Transfer or RAFT polymerization is one kind of controlled radical polymerization. Discovered at the Commonwealth Scientific and Industrial Research Organisation in 1998, RAFT polymerization is a relatively new method for the synthesis of living radical...
process, also termed MADIX (macromolecular design via interchange of xanthates).
Related compounds
Rarely encountered, thioxanthates arise by the reaction of CS2 with thiolate salts. For example, sodium ethylthioxanthate has the formula C2H5SCS2Na. DithiocarbamateDithiocarbamate
A dithiocarbamate is a functional group in organic chemistry. It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms. Sodium diethyldithiocarbamate is a common ligand in inorganic chemistry....
s are also related compounds, and are more useful. They arise from the reaction of the amine with CS2. For example, sodium diethyldithiocarbamate
Sodium diethyldithiocarbamate
Sodium diethyldithiocarbamate is the organosulfur compound with the formula NaS2CN2.-Preparation:This salt is obtained by treating carbon disulfide with diethylamine in the presence of sodium hydroxide:Other dithiocarbamates can be prepared similarly from secondary amines and carbon disulfide...
has the formula (C2H5)2NCS2Na.

