_chloride.gif)
Uranium(III) chloride
Encyclopedia
Uranium chloride , UCl3, is a chemical compound
that contains the earth metal uranium
and chlorine
. UCl3 is used mostly to reprocess spent nuclear fuel. Uranium(III) chloride is synthesized various ways from uranium(IV) chloride; however, UCl3 is less stable than UCl4.
(1) In a mixture of NaCl-KCl at 670-710 °C, add uranium tetrachloride with uranium metal.
(2) Heat uranium(IV) chloride in hydrogen gas.
 Uranium(III) chloride is a green crystalline solid at room temperature. UCl3 melts at 837 °C and boils at 1657 °C. Uranium(III) chloride has a density of 5500 kg/m³ or 5.500 g/cm³.
Uranium(III) chloride is a green crystalline solid at room temperature. UCl3 melts at 837 °C and boils at 1657 °C. Uranium(III) chloride has a density of 5500 kg/m³ or 5.500 g/cm³.
Its composition by weight:
Its formal oxidative states:
Uranium(III) chloride is very soluble in water and is also very hygroscopic. UCl3 is more stable in a solution of hydrochloric acid
.
(THF) and sodium methylcyclopentadiene to prepare various uranium metallocene
complexes.
(LiAlH4) and olefins to produce alkyl aluminate compounds.
Each are synthesized by the reduction of uranium(IV) chloride in methylcyanide (acetonitrile
), with specific amounts of water and propionic acid
.
Similar to other uranium compounds that are soluble, UCl3 is likely absorbed into the blood through the alveolar pockets of the lungs within days of exposure. Exposure to uranium(III) chloride leads to toxicity of the renal system
.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
that contains the earth metal uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
and chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
. UCl3 is used mostly to reprocess spent nuclear fuel. Uranium(III) chloride is synthesized various ways from uranium(IV) chloride; however, UCl3 is less stable than UCl4.
Preparation
There are two ways to synthesize uranium(III) chloride. The following processes describe how to produce uranium(III) chloride.(1) In a mixture of NaCl-KCl at 670-710 °C, add uranium tetrachloride with uranium metal.
- 3UCl4 + UUraniumUranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
→ 4UCl3
(2) Heat uranium(IV) chloride in hydrogen gas.
- 2UCl4 + H2HydrogenHydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
→ 2UCl3 + 2HClHydrochloric acidHydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
Properties
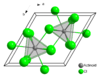
In solid uranium(III) chloride each uranium atom has nine chlorine atoms as near neighbours, at approximately the same distance, in a tricapped trigonal prismatic configuration.
Its composition by weight:
- Chlorine: 30.84%
- Uranium: 69.16%
Its formal oxidative states:
- Chlorine: −1
- Uranium: +3
Uranium(III) chloride is very soluble in water and is also very hygroscopic. UCl3 is more stable in a solution of hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
.
Reagent
Uranium(III) chloride is used in reactions with tetrahydrofuranTetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
(THF) and sodium methylcyclopentadiene to prepare various uranium metallocene
Metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions bound to a metal center in the oxidation state II, with the resulting general formula 2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride, vanadocene dichloride...
complexes.
Catalyst
Uranium(III) chloride is used as a catalyst during reactions between lithium aluminium hydrideLithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
(LiAlH4) and olefins to produce alkyl aluminate compounds.
Molten form
The molten form of uranium(III) chloride is a typical compound in pyrochemical processes as it is important in the reprocessing of spent nuclear fuels.UCl3 is usually the form that uranium takes as spent fuel in electrorefining processes.,Hydrates
There are three hydrates of uranium(III) chloride:- UCl3.2H2O.2CH3CN
- UCl3.6H2O
- UCl3.7H2O
Each are synthesized by the reduction of uranium(IV) chloride in methylcyanide (acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
), with specific amounts of water and propionic acid
Propionic acid
Propanoic acid is a naturally occurring carboxylic acid with chemical formula CH3CH2COOH. It is a clear liquid with a pungent odor...
.
Precautions
While there are no long-term data on the toxic effects thas UCl3, it is important to minimize exposure to this compound when possible.Similar to other uranium compounds that are soluble, UCl3 is likely absorbed into the blood through the alveolar pockets of the lungs within days of exposure. Exposure to uranium(III) chloride leads to toxicity of the renal system
Nephrotoxicity
Nephrotoxicity is a poisonous effect of some substances, both toxic chemicals and medication, on the kidneys. There are various forms of toxicity. Nephrotoxicity should not be confused with the fact that some medications have a predominantly renal excretion and need their dose adjusted for the...
.

