
Thermochemical cycle
Encyclopedia
Thermochemical cycles combine solely heat sources (thermo) with chemical reactions to split water
into its hydrogen
and oxygen
components . The term cycle is used because aside of water, hydrogen and oxygen, the chemical compounds used in these processes are continuously recycled.
If work
is partially used as an input, the resulting thermochemical cycle is defined as a hybrid one.
, ammonia
) from stable and abundant species (e.g. water
, nitrogen
) and heat sources . Although fuel availability was scarcely considered before the oil crisis
era, these researches were justified by niche market
s. As an example, in the military logistics
field, providing fuels for vehicles in remote battlefields is a key task. Hence, a mobile production system based on a portable heat source (a specific nuclear reactor
was strikingly considered) was being investigated with the uttermost interest.
Following the crisis, many programs (Europe,Japan,USA) were set up to design, test and qualify such processes for more peaceful purposes such as energy independence. High temperature (1000K) nuclear reactors were still considered as the heat sources. However, the optimistic expectations of the first thermodynamics studies were quickly moderated by more pragmatic analysis based on fair comparisons with standard technologies (thermodynamic cycles for electricity generation, coupled with the electrolysis of water
) and by numerous practical issues (not high enough temperatures with nuclear reactors, slow reactivities, reactor corrosion, significant losses of intermediate compounds with time...) . Hence, the interest for this technology was fading away during the next decades, or at least some tradeoffs (hybrid versions) were being considered with the use of electricity as a fractional energy input instead of only heat for the reactions (e.g. Hybrid sulfur cycle
). A rebirth in the year 2000 can be explained by both new energy crisis and the rapid pace of development of concentrated solar power technologies whose potentially very high temperatures are ideal for thermochemical processes , while the environmentally friendly
side of these researches attracts funding in a period with the peak oil
shadow.
at constant pressure and thermodynamic temperature
T:
Equilibrium is displaced to the right only if energy (enthalpy
change ΔH for water-splitting) is provided to the system under strict conditions imposed by Thermodynamics
:
Hence, for an ambient temperature T° of 298K (kelvin
) and a pressure of 1 atm (atmosphere (unit)
) (ΔG° and ΔS° are respectively equal to 237 kJ/mol and 163 J/mol/K, relative to the initial amount of water), more than 80% of the required energy ΔH must be provided as work in order for water-splitting to proceed.
If phase transitions are neglected for simplicity's sake (e.g. water electrolysis under pressure to keep water in its liquid state), one can assume that ΔH et ΔS do not vary significantly for a given temperature change. These parameters are thus taken equal to their standard values ΔH° et ΔS° at temperature T°. Consequently, the work required at temperature T is,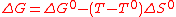 (3)
(3)
As ΔS° is positive, a temperature increase leads to a reduction of the required work. This is the basis of high-temperature electrolysis
. This can also be intuitively explained graphically.
Chemical species can have various excitation levels depending on the absolute temperature T, which is a measure of the thermal agitation. The latter causes shocks between atoms or molecules inside the closed system such that energy spreading among the excitation levels increases with time, and stop (equilibrium) only when most of the species have similar excitation levels (a molecule in a highly excited level will quickly return to a lower energy state by collisions) (Entropy (statistical thermodynamics)).
Relative to the absolute temperature scale, the excitation levels of the species are gathered based on standard enthalpy change of formation
considerations; i.e. their stabilities. As this value is null for water but strictly positive for oxygen and hydrogen, most of the excitation levels of these last species are above the ones of water. Then, the density of the excitation levels for a given temperature range is monotonically increasing with the species entropy. A positive entropy change for water-splitting means far more excitation levels in the products. Consequently,
One can imagine that if T were high enough in Eq.(3), ΔG could be nullified, meaning that water-splitting would occur even without work (thermolysis of water). Though possible, this would required tremendously high temperatures: considering the same system naturally with steam instead of liquid water (ΔH° = 242 kJ/mol; ΔS° = 44 J/mol/K) would hence give required temperatures above 3000K, that make reactor design and operation extremely challenging.
Hence, a single reaction only offers one freedom degree (T) to produce hydrogen and oxygen only from heat (though using Le Chatelier's principle
would also allow to slightly decrease the thermolysis temperature, work must be provided in this case for extracting the gas products from the system)
The first pre-requisites (Eqs.(4) and (5)) for multiple reactions i to be equivalent to water-splitting are trivial (cf. Hess's law
):
Similarly, the work ΔG required by the process is the sum of each reaction work ΔGi:
 (6)
(6)
As Eq.(3) is a general law, it can be used anew to develop each ΔGi term. If the reactions with positive (p indice) and negative (n indice) entropy changes are expressed as separate summations, this gives,
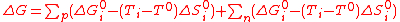 (7)
(7)
Using Eq.(6) for standard conditions allows to factorize the ΔG°i terms, yielding,
 (8)
(8)
Now let us consider the contribution of each summation in Eq.(8): in order to minimize ΔG, they must be as negative as possible:
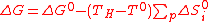 (9)
(9)
Finally, one can deduce from this last equation the relationship required for a null work requirement (ΔG ≤ 0)
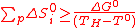 (10)
(10)
Consequently, a thermochemical cycle with i steps can be defined as sequence of i reactions equivalent to water-splitting and satisfying equations (4), (5) and (10). The key point to remember in that case is that the process temperature TH can theoretically be arbitrary chosen (1000K as a reference in most of the past studies, for high temperature nuclear reactors), far below the water thermolysis one.
This equation can alternatively (and naturally) be derived via the Carnot's theorem
, that must be respected by the system composed of a thermochemical process coupled with a work producing unit (chemical species are thus in a closed loop):
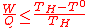 (11)
(11)
Consequently, replacing W (ΔG°) and Q (Eq.(14)) in Eq.(11) gives after reorganization Eq.(10) (assuming that the ΔSi do not change significantly with the temperature, i.e. are equal to ΔS°i)
Equation (10) has practical implications about the minimum number of reactions for such a process according to the maximum process temperature TH. Indeed, a numerical application (ΔG° equals to 229 kJ/K for water considered as steam) in the case of the originally chosen conditions (high-temperature nuclear reactor with TH and T° respectively equal to 1000K and 298K) gives a minimum value around 330 J/mol/K for the summation of the positive entropy changes ΔS°i of the process reactions.
This last value is very high as most of the reactions have entropy change values below 50 J/mol/K, and even an elevated one (e.g. water-splitting from liquid water: 163 J/mol/K) is twice lower. Consequently, thermochemical cycles composed of less than three steps are practically impossible with the originally planned heat sources (below 1000K), or require "hybrid" versions
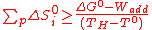 (15)
(15)
If Wadd is expressed as a fraction f of the process heat Q (Eq.(14)), Eq.(15) becomes after reorganization,
 (16)
(16)
Using a work input equals to a fraction f of the heat input is equivalent relative to the choice of the reactions to operate a pure similar thermochemical cycle but with a hot source with a temperature increased by the same proportion f.
Naturally, this decreases the heat-to-work efficiency in the same proportion f. Consequently, if one want a process similar to a thermochemical cycle operating with a 2000K heat source (instead of 1000K), the maximum heat-to-work efficiency is twice lower. As real efficiencies are often significantly lower than ideal one, such a process is thus strongly limited.
Practically, use of work is restricted to key steps such as product separations, where techniques relying on work (e.g. electrolysis) might sometimes have fewer issues than those using only heat (e.g. distillation
s)
s. As an example in Europe, this is the goal of the Hydrosol-2
project (Greece, Germany (German Aerospace Center
), Spain, Denmark, England) and of the researches of the solar department of the ETH Zurich
and the Paul Scherrer Institute
(Switzerland).
Examples of reactions satisfying high entropy changes are metal oxide dissociation
s, as the products have more excitation levels due to their gaseous state (metal vapors and oxygen) than the reactant (solid with crystalline structure, so symmetry dramatically reduces the number of different excitation levels). Consequently, these entropy changes can often be larger than the water-splitting one and thus a reaction with a negative entropy change is required in the thermochemical process so that Eq.(5) is satisfied. Furthermore, assuming similar stabilities of the reactant (ΔH°) for both thermolysis and oxide dissociation, a larger entropy change in the second case explained again a lower reaction temperature (Eq.(3)).
Let us assume two reactions, with positive (1 subscript, at TH) and negative (2 subscript, at T°) entropy changes. An extra property can be derived in order to have TH strictly lower than the thermolysis temperature: The standard thermodynamic values must be unevenly distributed among the reactions .
Indeed, according to the general equations (2) (spontaneous reaction), (4) and (5), one must satisfy,
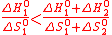 (17)
(17)
Hence, if ΔH°1 is proportional to ΔH°2 by a given factor, and if ΔS°1 and ΔS°2 follow a similar law (same proportionality factor), the inequality (17) is broken (equality instead, so TH equals to the water thermolysis temperature).
chemical element
is its high covalence. Indeed, it can form up to 6 chemical bonds with other elements such as oxygen (e.g. sulfates), i.e. a wide range of oxidation states. Hence, there exist several redox
reactions involving such compounds. This freedom allows numerous chemical steps with different entropy changes, and thus offer more odds to meet the criteria required for a thermochemical cycle (cf. Principles). Most of the first studies were performed in the USA, as an example at the Kentucky University for sulfide-bases cycles . Sulfate-based cycles were studied in the same laboratory and also at Los Alamos National Laboratory
and at General Atomics
. Significant researches based on sulfates (e.g. FeSO4 and CuSO4) were also performed in Germany and in Japan
. However, the cycle which has given rise to the highest interests is probably the (Sulfur-iodine cycle
) one (acronym: S-I) discovered by General Atomics .
Cycles based on the reversed Deacon process
Above 973K, the Deacon reaction is reversed, yielding hydrogen chloride
and oxygen from water and chlorine
:
Work in progress...
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
into its hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
components . The term cycle is used because aside of water, hydrogen and oxygen, the chemical compounds used in these processes are continuously recycled.
If work
Work (thermodynamics)
In thermodynamics, work performed by a system is the energy transferred to another system that is measured by the external generalized mechanical constraints on the system. As such, thermodynamic work is a generalization of the concept of mechanical work in mechanics. Thermodynamic work encompasses...
is partially used as an input, the resulting thermochemical cycle is defined as a hybrid one.
History
This concept was first postulated by Funk and Reinstrom (1966) as a reflexion about the most efficient way to produce fuels (e.g. hydrogenHydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
) from stable and abundant species (e.g. water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
) and heat sources . Although fuel availability was scarcely considered before the oil crisis
Oil crisis
Oil crisis may refer to:1970s*1970s energy crisis*1973 oil crisis*1979 energy crisisPost 1970s*Oil price increase of 1990*2000s energy crisis...
era, these researches were justified by niche market
Niche market
A niche market is the subset of the market on which a specific product is focusing; therefore the market niche defines the specific product features aimed at satisfying specific market needs, as well as the price range, production quality and the demographics that is intended to impact...
s. As an example, in the military logistics
Military logistics
Military logistics is the discipline of planning and carrying out the movement and maintenance of military forces. In its most comprehensive sense, it is those aspects or military operations that deal with:...
field, providing fuels for vehicles in remote battlefields is a key task. Hence, a mobile production system based on a portable heat source (a specific nuclear reactor
Nuclear power
Nuclear power is the use of sustained nuclear fission to generate heat and electricity. Nuclear power plants provide about 6% of the world's energy and 13–14% of the world's electricity, with the U.S., France, and Japan together accounting for about 50% of nuclear generated electricity...
was strikingly considered) was being investigated with the uttermost interest.
Following the crisis, many programs (Europe,Japan,USA) were set up to design, test and qualify such processes for more peaceful purposes such as energy independence. High temperature (1000K) nuclear reactors were still considered as the heat sources. However, the optimistic expectations of the first thermodynamics studies were quickly moderated by more pragmatic analysis based on fair comparisons with standard technologies (thermodynamic cycles for electricity generation, coupled with the electrolysis of water
Electrolysis of water
Electrolysis of water is the decomposition of water into oxygen and hydrogen gas due to an electric current being passed through the water.-Principle:...
) and by numerous practical issues (not high enough temperatures with nuclear reactors, slow reactivities, reactor corrosion, significant losses of intermediate compounds with time...) . Hence, the interest for this technology was fading away during the next decades, or at least some tradeoffs (hybrid versions) were being considered with the use of electricity as a fractional energy input instead of only heat for the reactions (e.g. Hybrid sulfur cycle
Hybrid sulfur cycle
The hybrid sulfur cycle is a two-step water-splitting process intended to be used for hydrogen production. Based on sulfur oxidation and reduction, it is classified as a hybrid thermochemical cycle because it uses an electrochemical reaction for one of the two steps...
). A rebirth in the year 2000 can be explained by both new energy crisis and the rapid pace of development of concentrated solar power technologies whose potentially very high temperatures are ideal for thermochemical processes , while the environmentally friendly
Environmentally friendly
Environmentally friendly are terms used to refer to goods and services, laws, guidelines and policies claimed to inflict minimal or no harm on the environment....
side of these researches attracts funding in a period with the peak oil
Peak oil
Peak oil is the point in time when the maximum rate of global petroleum extraction is reached, after which the rate of production enters terminal decline. This concept is based on the observed production rates of individual oil wells, projected reserves and the combined production rate of a field...
shadow.
Water-splitting via a single reaction
Let us consider a system composed of chemical species (e.g. water-splitting) in thermodynamic equilibriumThermodynamic equilibrium
In thermodynamics, a thermodynamic system is said to be in thermodynamic equilibrium when it is in thermal equilibrium, mechanical equilibrium, radiative equilibrium, and chemical equilibrium. The word equilibrium means a state of balance...
at constant pressure and thermodynamic temperature
Thermodynamic temperature
Thermodynamic temperature is the absolute measure of temperature and is one of the principal parameters of thermodynamics. Thermodynamic temperature is an "absolute" scale because it is the measure of the fundamental property underlying temperature: its null or zero point, absolute zero, is the...
T:
-
-
-
-
-
-
-
-
-
-
- H2O(l)
 H2(g) + 1/2 O2(g) (1)
H2(g) + 1/2 O2(g) (1)
- H2O(l)
-
-
-
-
-
-
-
-
-
Equilibrium is displaced to the right only if energy (enthalpy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
change ΔH for water-splitting) is provided to the system under strict conditions imposed by Thermodynamics
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
:
- one fraction must be provided as workWork (thermodynamics)In thermodynamics, work performed by a system is the energy transferred to another system that is measured by the external generalized mechanical constraints on the system. As such, thermodynamic work is a generalization of the concept of mechanical work in mechanics. Thermodynamic work encompasses...
, namely the Gibbs free energyGibbs free energyIn thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
change ΔG of the reaction: it consists of "noble" energy, i.e. under an organized state where matter can be controlled, such as electricity in the case of the electrolysis of waterElectrolysis of waterElectrolysis of water is the decomposition of water into oxygen and hydrogen gas due to an electric current being passed through the water.-Principle:...
. Indeed, the generated electron flow can reduce protons (H+) ) at the cathode and oxidize anions (O2−) at the anode (the ions exist because of the chemical polarityChemical polarityIn chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
of water), yielding the desired species. - the other one must be supplied as heatHeatIn physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
, i.e. by increasing the thermal agitation of the species, and is equal by definition of the entropyEntropyEntropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
to the absolute temperature T times the entropy change ΔS of the reaction.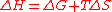 (2)
(2)
Hence, for an ambient temperature T° of 298K (kelvin
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
) and a pressure of 1 atm (atmosphere (unit)
Atmosphere (unit)
The standard atmosphere is an international reference pressure defined as 101325 Pa and formerly used as unit of pressure. For practical purposes it has been replaced by the bar which is 105 Pa...
) (ΔG° and ΔS° are respectively equal to 237 kJ/mol and 163 J/mol/K, relative to the initial amount of water), more than 80% of the required energy ΔH must be provided as work in order for water-splitting to proceed.
If phase transitions are neglected for simplicity's sake (e.g. water electrolysis under pressure to keep water in its liquid state), one can assume that ΔH et ΔS do not vary significantly for a given temperature change. These parameters are thus taken equal to their standard values ΔH° et ΔS° at temperature T°. Consequently, the work required at temperature T is,
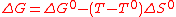 (3)
(3)As ΔS° is positive, a temperature increase leads to a reduction of the required work. This is the basis of high-temperature electrolysis
High-temperature electrolysis
High-temperature electrolysis is a method currently being investigated for the production of hydrogen from water with oxygen as a by-product.-Efficiency:...
. This can also be intuitively explained graphically.
Chemical species can have various excitation levels depending on the absolute temperature T, which is a measure of the thermal agitation. The latter causes shocks between atoms or molecules inside the closed system such that energy spreading among the excitation levels increases with time, and stop (equilibrium) only when most of the species have similar excitation levels (a molecule in a highly excited level will quickly return to a lower energy state by collisions) (Entropy (statistical thermodynamics)).
Relative to the absolute temperature scale, the excitation levels of the species are gathered based on standard enthalpy change of formation
Standard enthalpy change of formation
The standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy that accompanies the formation of 1 mole of a substance in its standard state from its constituent elements in their standard states...
considerations; i.e. their stabilities. As this value is null for water but strictly positive for oxygen and hydrogen, most of the excitation levels of these last species are above the ones of water. Then, the density of the excitation levels for a given temperature range is monotonically increasing with the species entropy. A positive entropy change for water-splitting means far more excitation levels in the products. Consequently,
- A low temperature (T°), thermal agitation allow mostly the water molecules to be excited as hydrogen and oxygen levels required higher thermal agitation to be significantly populated (on the arbitrary diagram, 3 levels can be populated for water vs 1 for the oxygen/hydrogen subsystem),
- At high temperature (T), thermal agitation is sufficient for the oxygen/hydrogen subsystem excitation levels to be excited (on the arbitrary diagram, 4 levels can be populated for water vs 8 for the oxygen/hydrogen subsystem). According to the previous statements, the system will thus evolve toward the composition where most of its excitation levels are similar, i.e. a majority of oxygen and hydrogen species.
One can imagine that if T were high enough in Eq.(3), ΔG could be nullified, meaning that water-splitting would occur even without work (thermolysis of water). Though possible, this would required tremendously high temperatures: considering the same system naturally with steam instead of liquid water (ΔH° = 242 kJ/mol; ΔS° = 44 J/mol/K) would hence give required temperatures above 3000K, that make reactor design and operation extremely challenging.
Hence, a single reaction only offers one freedom degree (T) to produce hydrogen and oxygen only from heat (though using Le Chatelier's principle
Le Châtelier's principle
In chemistry, Le Chatelier's principle, also called the Chatelier's principle, can be used to predict the effect of a change in conditions on a chemical equilibrium. The principle is named after Henry Louis Le Chatelier and sometimes Karl Ferdinand Braun who discovered it independently...
would also allow to slightly decrease the thermolysis temperature, work must be provided in this case for extracting the gas products from the system)
Water-splitting with multiple reactions
On the contrary, as shown by Funk and Reinstrom, multiple reactions (e.g. k steps) provide additional means to allow spontaneous water-splitting without work thanks to different entropy changes ΔS°i for each reaction i. An extra benefit compared with water thermolysis is that oxygen and hydrogen are separately produced, avoiding complex separations at high temperatures.The first pre-requisites (Eqs.(4) and (5)) for multiple reactions i to be equivalent to water-splitting are trivial (cf. Hess's law
Hess's law
Hess' law is a relationship in physical chemistry named for Germain Hess, a Swiss-born Russian chemist and physician.The law states that the enthalpy change for a reaction that is carried out in a series of steps is equal to the sum of the enthalpy changes for the individual steps.The law is an...
):
-
 (4)
(4)
-
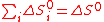 (5)
(5)
Similarly, the work ΔG required by the process is the sum of each reaction work ΔGi:
 (6)
(6)As Eq.(3) is a general law, it can be used anew to develop each ΔGi term. If the reactions with positive (p indice) and negative (n indice) entropy changes are expressed as separate summations, this gives,
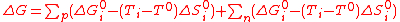 (7)
(7)Using Eq.(6) for standard conditions allows to factorize the ΔG°i terms, yielding,
 (8)
(8)Now let us consider the contribution of each summation in Eq.(8): in order to minimize ΔG, they must be as negative as possible:
-
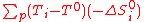 : -ΔS°i are negative, so (T-T°) must be as high as possible: hence, one choose to operate at the maximum process temperature TH
: -ΔS°i are negative, so (T-T°) must be as high as possible: hence, one choose to operate at the maximum process temperature TH -
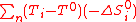 : -ΔS°i are positive, (T-T°) should be ideally negative in order to decrease ΔG. Practically, one can only set T equals to T° as the minimum process temperature in order to get rid of this troublesome term (a process requiring a lower than standard temperature for energy production is a physical absurdity as it would required refrigerators and thus a higher work input than output). Consequently, Eq.(8) becomes,
: -ΔS°i are positive, (T-T°) should be ideally negative in order to decrease ΔG. Practically, one can only set T equals to T° as the minimum process temperature in order to get rid of this troublesome term (a process requiring a lower than standard temperature for energy production is a physical absurdity as it would required refrigerators and thus a higher work input than output). Consequently, Eq.(8) becomes,
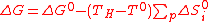 (9)
(9)Finally, one can deduce from this last equation the relationship required for a null work requirement (ΔG ≤ 0)
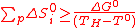 (10)
(10)Consequently, a thermochemical cycle with i steps can be defined as sequence of i reactions equivalent to water-splitting and satisfying equations (4), (5) and (10). The key point to remember in that case is that the process temperature TH can theoretically be arbitrary chosen (1000K as a reference in most of the past studies, for high temperature nuclear reactors), far below the water thermolysis one.
This equation can alternatively (and naturally) be derived via the Carnot's theorem
Carnot's theorem
In Euclidean geometry, Carnot's theorem, named after Lazare Carnot , is as follows. Let ABC be an arbitrary triangle. Then the sum of the signed distances from the circumcenter D to the sides of triangle ABC is...
, that must be respected by the system composed of a thermochemical process coupled with a work producing unit (chemical species are thus in a closed loop):
- at least two heat sources of different temperatures are required for cyclical operation, otherwise perpetual motionPerpetual motionPerpetual motion describes hypothetical machines that operate or produce useful work indefinitely and, more generally, hypothetical machines that produce more work or energy than they consume, whether they might operate indefinitely or not....
would be possible. This is trivial in the case of thermolysis, as the fuel is consumed via an inverse reaction. Consequently, if there is only one temperature (the thermolysis one), maximum work recovery in a fuel cell is equal to the opposite of the Gibbs free energy of the water-splitting reaction at the same temperature, i.e. null by definition of the thermolysis. Or differently said, a fuel is defined by its instability, so if the water/hydrogen/oxygen system only exists as hydrogen and oxygen (equilibrium state), combustion (engine) or use in a fuel cellFuel cellA fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
would not be possible. - endothermic reactions are chosen with positive entropy changes in order to be favored when the temperature increases, and the opposite for the exothermic reactions.
- maximal heat-to-work efficiency is the one of a Carnot heat engineCarnot heat engineA Carnot heat engine is a hypothetical engine that operates on the reversible Carnot cycle. The basic model for this engine was developed by Nicolas Léonard Sadi Carnot in 1824...
with the same process conditions, i.e. a hot heat source at TH and a cold one at T°,
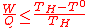 (11)
(11)- - the work output W is the "noble" energy stored in the hydrogen and oxygen products (e.g. released as electricity during fuel consumption in a fuel cellFuel cellA fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
). It thus corresponds to the free Gibbs energy change of water-splitting ΔG, and is maximum according to Eq.(3) at the lowest temperature of the process (T°) where it is equal to ΔG°. - - the heat input Q is the heat provided by the hot source at temperature TH to the i endothermicEndothermicIn thermodynamics, the word endothermic describes a process or reaction in which the system absorbs energy from the surroundings in the form of heat. Its etymology stems from the prefix endo- and the Greek word thermasi,...
reactions of the thermochemical cycle (the fuel consumption subsystem is exothermicExothermicIn thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
):-
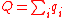 (12)
(12)
- Hence, each heat requirement at temperature TH is,
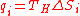 (13)
(13)
- Replacing Eq.(13) in Eq.(12) yields:
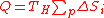 (14)
(14)
-
Consequently, replacing W (ΔG°) and Q (Eq.(14)) in Eq.(11) gives after reorganization Eq.(10) (assuming that the ΔSi do not change significantly with the temperature, i.e. are equal to ΔS°i)
Equation (10) has practical implications about the minimum number of reactions for such a process according to the maximum process temperature TH. Indeed, a numerical application (ΔG° equals to 229 kJ/K for water considered as steam) in the case of the originally chosen conditions (high-temperature nuclear reactor with TH and T° respectively equal to 1000K and 298K) gives a minimum value around 330 J/mol/K for the summation of the positive entropy changes ΔS°i of the process reactions.
This last value is very high as most of the reactions have entropy change values below 50 J/mol/K, and even an elevated one (e.g. water-splitting from liquid water: 163 J/mol/K) is twice lower. Consequently, thermochemical cycles composed of less than three steps are practically impossible with the originally planned heat sources (below 1000K), or require "hybrid" versions
Hybrid thermochemical cycles
In this case, an extra freedom degree is added via a relatively small work input Wadd (maximum work consumption, Eq.(9) with ΔG ≤ Wadd), and Eq.(10) becomes,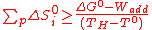 (15)
(15)If Wadd is expressed as a fraction f of the process heat Q (Eq.(14)), Eq.(15) becomes after reorganization,
 (16)
(16)Using a work input equals to a fraction f of the heat input is equivalent relative to the choice of the reactions to operate a pure similar thermochemical cycle but with a hot source with a temperature increased by the same proportion f.
Naturally, this decreases the heat-to-work efficiency in the same proportion f. Consequently, if one want a process similar to a thermochemical cycle operating with a 2000K heat source (instead of 1000K), the maximum heat-to-work efficiency is twice lower. As real efficiencies are often significantly lower than ideal one, such a process is thus strongly limited.
Practically, use of work is restricted to key steps such as product separations, where techniques relying on work (e.g. electrolysis) might sometimes have fewer issues than those using only heat (e.g. distillation
Distillation
Distillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction....
s)
Particular case : Two-step thermochemical cycles
According to equation (10), the minimum required entropy change (right term) for the summation of the positive entropy changes decreases when TH increases. As an example, performing the same numerical application but with TH equals to 2000K would give a twice lower value (around 140 kJ/mol), which allows thermochemical cycles with only two reactions. Such processes can be realistically coupled with concentrated solar power technologies like solar towerSolar tower
A solar tower, in the context of astronomy, is a structure used to support equipment for studying the sun, and is typically part of solar telescope designs. Generically, the term solar tower has many more uses especially for a type of power production using Earth's Sun...
s. As an example in Europe, this is the goal of the Hydrosol-2
Hydrosol-2
HYDROSOL is series of European Union funded projects for the promotion of renewable energy...
project (Greece, Germany (German Aerospace Center
German Aerospace Center
The German Aerospace Center is the national centre for aerospace, energy and transportation research of the Federal Republic of Germany. It has multiple locations throughout Germany. Its headquarters are located in Cologne. It is engaged in a wide range of research and development projects in...
), Spain, Denmark, England) and of the researches of the solar department of the ETH Zurich
ETH Zurich
The Swiss Federal Institute of Technology Zurich or ETH Zürich is an engineering, science, technology, mathematics and management university in the City of Zurich, Switzerland....
and the Paul Scherrer Institute
Paul Scherrer Institute
The Paul Scherrer Institute is a multi-disciplinary research institute which belongs to the Swiss ETH-Komplex covering also the ETH Zurich and EPFL...
(Switzerland).
Examples of reactions satisfying high entropy changes are metal oxide dissociation
Dissociation
Dissociation is an altered state of consciousness characterized by partial or complete disruption of the normal integration of a person’s normal conscious or psychological functioning. Dissociation is most commonly experienced as a subjective perception of one's consciousness being detached from...
s, as the products have more excitation levels due to their gaseous state (metal vapors and oxygen) than the reactant (solid with crystalline structure, so symmetry dramatically reduces the number of different excitation levels). Consequently, these entropy changes can often be larger than the water-splitting one and thus a reaction with a negative entropy change is required in the thermochemical process so that Eq.(5) is satisfied. Furthermore, assuming similar stabilities of the reactant (ΔH°) for both thermolysis and oxide dissociation, a larger entropy change in the second case explained again a lower reaction temperature (Eq.(3)).
Let us assume two reactions, with positive (1 subscript, at TH) and negative (2 subscript, at T°) entropy changes. An extra property can be derived in order to have TH strictly lower than the thermolysis temperature: The standard thermodynamic values must be unevenly distributed among the reactions .
Indeed, according to the general equations (2) (spontaneous reaction), (4) and (5), one must satisfy,
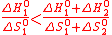 (17)
(17)Hence, if ΔH°1 is proportional to ΔH°2 by a given factor, and if ΔS°1 and ΔS°2 follow a similar law (same proportionality factor), the inequality (17) is broken (equality instead, so TH equals to the water thermolysis temperature).
Examples
Hundreds of such cycles have been proposed and investigated. This task has been eased by the availability of computers, allowing a systematic screening of chemical reactions sequences based on thermodynamic databases . Only the main "families" will be described in this article .Cycles based on the sulfur chemistry
An advantage of the sulfurSulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
chemical element
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
is its high covalence. Indeed, it can form up to 6 chemical bonds with other elements such as oxygen (e.g. sulfates), i.e. a wide range of oxidation states. Hence, there exist several redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
reactions involving such compounds. This freedom allows numerous chemical steps with different entropy changes, and thus offer more odds to meet the criteria required for a thermochemical cycle (cf. Principles). Most of the first studies were performed in the USA, as an example at the Kentucky University for sulfide-bases cycles . Sulfate-based cycles were studied in the same laboratory and also at Los Alamos National Laboratory
Los Alamos National Laboratory
Los Alamos National Laboratory is a United States Department of Energy national laboratory, managed and operated by Los Alamos National Security , located in Los Alamos, New Mexico...
and at General Atomics
General Atomics
General Atomics is a nuclear physics and defense contractor headquartered in San Diego, California. General Atomics’ research into fission and fusion matured into competencies in related technologies, allowing the company to expand into other fields of research...
. Significant researches based on sulfates (e.g. FeSO4 and CuSO4) were also performed in Germany and in Japan
. However, the cycle which has given rise to the highest interests is probably the (Sulfur-iodine cycle
Sulfur-iodine cycle
The sulfur–iodine cycle is a three-step thermochemical cycle used to produce hydrogen.The S–I cycle consists of three chemical reactions whose net reactant is water and whose net products are hydrogen and oxygen. All other chemicals are recycled...
) one (acronym: S-I) discovered by General Atomics .
Cycles based on the reversed Deacon processDeacon processThe Deacon process was a secondary process used during the manufacture of alkalis by the Leblanc process. Hydrogen chloride gas was converted to chlorine gas which was then used to manufacture a commercially valuable bleaching powder, and at the same time the emission of waste hydrochloric acid...
Above 973K, the Deacon reaction is reversed, yielding hydrogen chlorideHydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
and oxygen from water and chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
:
-
- H2O + Cl2 → 2 HCl + 1/2 O2
Work in progress...
- Iron oxide cycleIron oxide cycleThe iron oxide cycle is a two-step thermochemical cycle proposed for use for hydrogen production.-Process description:The thermochemical two-step water splitting process uses redox systems...
- Cerium(IV) oxide-cerium(III) oxide cycleCerium(IV) oxide-cerium(III) oxide cycleThe cerium oxide–cerium oxide cycle or CeO2/Ce2O3 cycle is a two step thermochemical process based on cerium oxide and cerium oxide for hydrogen production...
- Copper-chlorine cycleCopper-chlorine cycleThe copper–chlorine cycle is a four-step thermochemical cycle. It has a maximum temperature requirement of about 530 degrees Celsius. The Cu–Cl cycle is one of the prominent thermochemical cycles under development within the Generation IV International Forum...
- Hybrid sulfur cycleHybrid sulfur cycleThe hybrid sulfur cycle is a two-step water-splitting process intended to be used for hydrogen production. Based on sulfur oxidation and reduction, it is classified as a hybrid thermochemical cycle because it uses an electrochemical reaction for one of the two steps...
- Hydrosol-2Hydrosol-2HYDROSOL is series of European Union funded projects for the promotion of renewable energy...
- Sulfur-iodine cycleSulfur-iodine cycleThe sulfur–iodine cycle is a three-step thermochemical cycle used to produce hydrogen.The S–I cycle consists of three chemical reactions whose net reactant is water and whose net products are hydrogen and oxygen. All other chemicals are recycled...
- Zinc zinc-oxide cycle
- UT-3 cycle

