Oxygen-haemoglobin dissociation curve
Encyclopedia
The oxygen–haemoglobin dissociation curve (or oxygen–hemoglobin dissociation curve) plots the proportion of haemoglobin
in its saturated form on the vertical axis against the prevailing oxygen
tension on the horizontal axis. The oxyhaemoglobin dissociation curve is an important tool for understanding how our blood carries and releases oxygen. Specifically, the oxyhaemoglobin dissociation curve relates oxygen saturation (SO2) and partial pressure of oxygen in the blood (PO2), and is determined by what is called "haemoglobin's affinity for oxygen"; that is, how readily haemoglobin acquires and releases oxygen molecules into the fluid that surrounds it.
The amount of oxygen bound to the haemoglobin at any time is related, in large part, to the partial pressure of oxygen to which the haemoglobin is exposed. In the lungs, at the alveolar–capillary interface, the partial pressure of oxygen is typically high, and therefore the oxygen binds readily to haemoglobin that is present. As the blood circulates to other body tissue in which the partial pressure of oxygen is less, the haemoglobin releases the oxygen into the tissue because the haemoglobin cannot maintain its full bound capacity of oxygen in the presence of lower oxygen partial pressures
 It is usually a sigmoid
It is usually a sigmoid
plot. A haemoglobin molecule can bind up to four oxygen molecules in a reversible way.
The shape of the curve results from the interaction of bound oxygen molecules with incoming molecules. The binding of the first molecule is difficult. However, this facilitates the binding of the second and third molecules, and it is only when the fourth molecule is to be bound that the difficulty increases, partly as a result of crowding of the haemoglobin molecule, partly as a natural tendency of oxygen to dissociate.
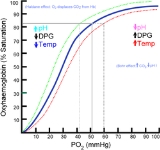
In its most simple form, the oxyhaemoglobin dissociation curve describes the relation between the partial pressure of oxygen (x axis) and the oxygen saturation (y axis). Haemoglobin's affinity for oxygen increases as successive molecules of oxygen bind. More molecules bind as the oxygen partial pressure increases until the maximum amount that can be bound is reached. As this limit is approached, very little additional binding occurs and the curve levels out as the haemoglobin becomes saturated with oxygen. Hence the curve has a sigmoidal or S-shape. At pressures above about 60 mmHg, the standard dissociation curve is relatively flat, which means that the oxygen content of the blood does not change significantly even with large increases in the oxygen partial pressure. To get more oxygen to the tissue would require blood transfusions to increase the haemoglobin count (and hence the oxygen-carrying capacity), or supplemental oxygen that would increase the oxygen dissolved in plasma.
Although binding of oxygen to haemoglobin continues to some extent for pressures about 50
mmHg, as oxygen partial pressures decrease in this steep area of the curve, the oxygen is unloaded to peripheral tissue readily as the haemoglobin's affinity diminishes.
The partial pressure of oxygen in the blood at which the haemoglobin is 50% saturated, typically about 26.6 mmHg for a healthy person, is known as the P50. The P50 is a conventional measure of haemoglobin affinity for oxygen. In the presence of disease or other conditions that change the haemoglobin's oxygen affinity and, consequently, shift the curve to the right or left, the P50 changes accordingly. An increased P50 indicates a rightward shift of the standard curve, which means that a larger partial pressure is necessary to maintain a 50% oxygen saturation. This indicates a decreased affinity. Conversely, a lower P50 indicates a leftward shift and a higher affinity.
The 'plateau' portion of the oxyhaemoglobin dissociation curve is the range that exists at the pulmonary capillaries (minimal reduction of oxygen transported until the p(O2) falls 50 mmHg).
The 'steep' portion of the oxyhaemoglobin dissociation curve is the range that exists at the systemic capillaries (a small drop in systemic capillary p(O2) can result in the release of large amounts of oxygen for the metabolically active cells).
To see the relative affinities of each successive oxygen as you remove/add oxygen from/to the haemoglobin from the curve compare the relative increase/decrease in p(O2) needed for the corresponding increase/decrease in s(O2).
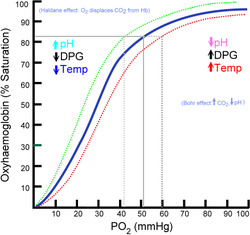
Left shift of the curve is a sign of haemoglobin's increased affinity for oxygen (e.g. at the lungs). Similarly, right shift shows decreased affinity, as would appear with an increase in body temperature, hydrogen ion, 2,3-diphosphoglycerate (also known as bisphosphoglycerate) or carbon dioxide concentration (the Bohr effect)
The causes of shift to right can be remembered using the mnemonic
, "CADET, face Right!" for CO2, Acid, 2,3-DPG, Exercise and Temperature.
Factors that move the oxygen dissociation curve to the right are those physiological states where tissues need more oxygen. For example during exercise, muscles have a higher metabolic rate, and consequently need more oxygen, produce more carbon dioxide and lactic acid, and their temperature rises.
. A reduction in the total binding capacity of haemoglobin to oxygen (i.e. shifting the curve down, not just to the right) due to reduced pH is called the root effect
. This is seen in bony fish.
affects the curve in two ways: first, it influences intracellular pH
(the Bohr effect), and second, CO2 accumulation causes carbamino compounds to be generated through chemical interactions, which bind to haemoglobin forming carbaminohaemoglobin
. Low levels of carbamino compounds have the effect of shifting the curve to the right, while higher levels cause a leftward shift. However, this isn't the overriding effect of CO2 accumulation. Only about 5–10% of the total CO2 content of blood is transported as carbamino compounds. Most of the CO2 content (80–90%) is transported as bicarbonate ions. The formation of a bicarbonate ion will release a proton into the plasma. Hence, the elevated CO2 content creates a respiratory acidosis and shifts the oxygen–haemoglobin dissociation curve to the right.
200-250 times more readily than with oxygen. The presence of carbon monoxide on one of the 4 haem
sites causes the oxygen on the other haem sites to bind with greater affinity. This makes it difficult for the haemoglobin to release oxygen to the tissues and has the effect of shifting the curve to the left (as well as downward, due to direct competitive effects of carbon monoxide). With an increased level of carbon monoxide, a person can suffer from severe tissue hypoxia while maintaining a normal pO2.
is a form of abnormal haemoglobin where ferrous (Fe2+), which is normally found in haemoglobin, is converted to the ferric (Fe3+) state. This causes a leftward shift in the curve as methaemoglobin does not unload O2 from Hb. However, methaemoglobin has increased affinity for cyanide, and is therefore useful in the treatment of cyanide poisoning.
. At the placenta there is a higher concentration of 2,3-DPG formed which binds more readily to adult haemoglobin and not to fetal haemoglobin. This causes the adult HbA to release more oxygen at the placenta to be taken up by the fetus and put onto HbF. 2,3-DPG binds readily to beta chains and does not readily bind to gamma chains, hence HbF is not affected by 2,3-DPG. This exemplifies why the curve needs to be shifted to the left in the fetus. Oxygen unloading needs to be easier and oxygen binding needs to be improved at the lower pressures of fetal systemic circulation (a left shit) to provide oxygen to the developing fetus. HbA can pass oxygen on from maternal circulation across the placenta to the fetal circulation because HbF has a higher affinity to bind at lower partial pressures. HbF then delivers that bound oxygen to tissues that have even lower partial pressures where it can be released.

Hemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
in its saturated form on the vertical axis against the prevailing oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
tension on the horizontal axis. The oxyhaemoglobin dissociation curve is an important tool for understanding how our blood carries and releases oxygen. Specifically, the oxyhaemoglobin dissociation curve relates oxygen saturation (SO2) and partial pressure of oxygen in the blood (PO2), and is determined by what is called "haemoglobin's affinity for oxygen"; that is, how readily haemoglobin acquires and releases oxygen molecules into the fluid that surrounds it.
Background
Haemoglobin (Hb), a globular protein, is the primary vehicle for transporting oxygen in the blood. Oxygen is also carried dissolved in the blood's plasma, but to a much lesser degree. Haemoglobin is contained in erythrocytes, more commonly referred to as red blood cells. Under certain conditions, oxygen bound to the haemoglobin is released into the blood's plasma and absorbed into the tissues. Each haemoglobin molecule has the capacity to carry four oxygen molecules. How much of that capacity is filled by oxygen at any time is called the oxygen saturation. Expressed as a percentage, the oxygen saturation is the ratio of the amount of oxygen bound to the haemoglobin, to the oxygen-carrying capacity of the haemoglobin. The oxygen-carrying capacity of haemoglobin is determined by the type of haemoglobin present in the blood.The amount of oxygen bound to the haemoglobin at any time is related, in large part, to the partial pressure of oxygen to which the haemoglobin is exposed. In the lungs, at the alveolar–capillary interface, the partial pressure of oxygen is typically high, and therefore the oxygen binds readily to haemoglobin that is present. As the blood circulates to other body tissue in which the partial pressure of oxygen is less, the haemoglobin releases the oxygen into the tissue because the haemoglobin cannot maintain its full bound capacity of oxygen in the presence of lower oxygen partial pressures
Sigmoidal shape

Sigmoid function
Many natural processes, including those of complex system learning curves, exhibit a progression from small beginnings that accelerates and approaches a climax over time. When a detailed description is lacking, a sigmoid function is often used. A sigmoid curve is produced by a mathematical...
plot. A haemoglobin molecule can bind up to four oxygen molecules in a reversible way.
The shape of the curve results from the interaction of bound oxygen molecules with incoming molecules. The binding of the first molecule is difficult. However, this facilitates the binding of the second and third molecules, and it is only when the fourth molecule is to be bound that the difficulty increases, partly as a result of crowding of the haemoglobin molecule, partly as a natural tendency of oxygen to dissociate.
In its most simple form, the oxyhaemoglobin dissociation curve describes the relation between the partial pressure of oxygen (x axis) and the oxygen saturation (y axis). Haemoglobin's affinity for oxygen increases as successive molecules of oxygen bind. More molecules bind as the oxygen partial pressure increases until the maximum amount that can be bound is reached. As this limit is approached, very little additional binding occurs and the curve levels out as the haemoglobin becomes saturated with oxygen. Hence the curve has a sigmoidal or S-shape. At pressures above about 60 mmHg, the standard dissociation curve is relatively flat, which means that the oxygen content of the blood does not change significantly even with large increases in the oxygen partial pressure. To get more oxygen to the tissue would require blood transfusions to increase the haemoglobin count (and hence the oxygen-carrying capacity), or supplemental oxygen that would increase the oxygen dissolved in plasma.
Although binding of oxygen to haemoglobin continues to some extent for pressures about 50
mmHg, as oxygen partial pressures decrease in this steep area of the curve, the oxygen is unloaded to peripheral tissue readily as the haemoglobin's affinity diminishes.
The partial pressure of oxygen in the blood at which the haemoglobin is 50% saturated, typically about 26.6 mmHg for a healthy person, is known as the P50. The P50 is a conventional measure of haemoglobin affinity for oxygen. In the presence of disease or other conditions that change the haemoglobin's oxygen affinity and, consequently, shift the curve to the right or left, the P50 changes accordingly. An increased P50 indicates a rightward shift of the standard curve, which means that a larger partial pressure is necessary to maintain a 50% oxygen saturation. This indicates a decreased affinity. Conversely, a lower P50 indicates a leftward shift and a higher affinity.
The 'plateau' portion of the oxyhaemoglobin dissociation curve is the range that exists at the pulmonary capillaries (minimal reduction of oxygen transported until the p(O2) falls 50 mmHg).
The 'steep' portion of the oxyhaemoglobin dissociation curve is the range that exists at the systemic capillaries (a small drop in systemic capillary p(O2) can result in the release of large amounts of oxygen for the metabolically active cells).
To see the relative affinities of each successive oxygen as you remove/add oxygen from/to the haemoglobin from the curve compare the relative increase/decrease in p(O2) needed for the corresponding increase/decrease in s(O2).
Factors that affect the standard dissociation curve
The strength with which oxygen binds to haemoglobin is affected by several factors. These factors shift or reshape the oxyhaemoglobin dissociation curve. A rightward shift indicates that the haemoglobin under study has a decreased affinity for oxygen. This makes it more difficult for haemoglobin to bind to oxygen (requiring a higher partial pressure of oxygen to achieve the same oxygen saturation), but it makes it easier for the haemoglobin to release oxygen bound to it. The effect of this rightward shift of the curve increases the partial pressure of oxygen in the tissues when it is most needed, such as during exercise, or haemorrhagic shock. In contrast, the curve is shifted to the left by the opposite of these conditions. This leftward shift indicates that the haemoglobin under study has an increased affinity for oxygen so that haemoglobin binds oxygen more easily, but unloads it more reluctantly.Left shift of the curve is a sign of haemoglobin's increased affinity for oxygen (e.g. at the lungs). Similarly, right shift shows decreased affinity, as would appear with an increase in body temperature, hydrogen ion, 2,3-diphosphoglycerate (also known as bisphosphoglycerate) or carbon dioxide concentration (the Bohr effect)
| left shift (high affinity for O2) | right shift (low affinity for O2) |
| decrease | >- | decrease | >- | decrease | >- | increase | >- | increase (alkalosis Alkalosis Alkalosis refers to a condition reducing hydrogen ion concentration of arterial blood plasma . Generally, alkalosis is said to occur when pH of the blood exceeds 7.45. The opposite condition is acidosis .-Causes:... ) |
acidosis Acidosis Acidosis is an increased acidity in the blood and other body tissue . If not further qualified, it usually refers to acidity of the blood plasma.... ) >- | type of haemoglobin Hemoglobin Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates... |
fetal haemoglobin Fetal hemoglobin Fetal hemoglobin, or foetal haemoglobin, is the main oxygen transport protein in the fetus during the last seven months of development in the uterus and in the newborn until roughly 6 months old... |
adult haemoglobin |
The causes of shift to right can be remembered using the mnemonic
Mnemonic
A mnemonic , or mnemonic device, is any learning technique that aids memory. To improve long term memory, mnemonic systems are used to make memorization easier. Commonly encountered mnemonics are often verbal, such as a very short poem or a special word used to help a person remember something,...
, "CADET, face Right!" for CO2, Acid, 2,3-DPG, Exercise and Temperature.
Factors that move the oxygen dissociation curve to the right are those physiological states where tissues need more oxygen. For example during exercise, muscles have a higher metabolic rate, and consequently need more oxygen, produce more carbon dioxide and lactic acid, and their temperature rises.
Variation of the hydrogen ion concentration
This changes the blood's pH. A decrease in pH shifts the standard curve to the right, while an increase shifts it to the left. This is known as the Bohr effectBohr effect
Bohr effect is a property of hemoglobin first described in 1904 by the Danish physiologist Christian Bohr , which states that an increasing concentration of protons and/or carbon dioxide will reduce the oxygen affinity of hemoglobin...
. A reduction in the total binding capacity of haemoglobin to oxygen (i.e. shifting the curve down, not just to the right) due to reduced pH is called the root effect
Root effect
The Root Effect is a physiological phenomenon that occurs in fish hemoglobin, named after its discoverer R. W. Root. It is the phenomenon where an increased proton or carbon dioxide concentration lowers hemoglobin's affinity and carrying capacity for oxygen . The Root effect is to be...
. This is seen in bony fish.
Effects of carbon dioxide
Carbon dioxideCarbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
affects the curve in two ways: first, it influences intracellular pH
Intracellular pH
All cells contain an intracellular fluid whose pH value is known as the intracellular pH . The pHi plays a critical role in the function of the cell, and close regulation is required for cells to survive...
(the Bohr effect), and second, CO2 accumulation causes carbamino compounds to be generated through chemical interactions, which bind to haemoglobin forming carbaminohaemoglobin
Carbaminohemoglobin
Carbaminohemoglobin is a compound of hemoglobin and carbon dioxide, and is one of the forms in which carbon dioxide exists in the blood. When carbon dioxide binds to hemoglobin, carbaminohemoglobin is formed, lowering hemoglobin's affinity for oxygen via the Haldane effect...
. Low levels of carbamino compounds have the effect of shifting the curve to the right, while higher levels cause a leftward shift. However, this isn't the overriding effect of CO2 accumulation. Only about 5–10% of the total CO2 content of blood is transported as carbamino compounds. Most of the CO2 content (80–90%) is transported as bicarbonate ions. The formation of a bicarbonate ion will release a proton into the plasma. Hence, the elevated CO2 content creates a respiratory acidosis and shifts the oxygen–haemoglobin dissociation curve to the right.
Effects of 2,3-DPG
2,3-Diphosphoglycerate or 2,3-DPG (also 2,3-bisphosphoglycerate or 2,3-BPG) is an organophosphate, which is created in erythrocytes during glycolysis. The production of 2,3-DPG is likely an important adaptive mechanism, because the production increases for several conditions in the presence of diminished peripheral tissue O2 availability, such as hypoxaemia, chronic lung disease, anaemia, and congestive heart failure, among others. High levels of 2,3-DPG shift the curve to the right, while low levels of 2,3-DPG cause a leftward shift, seen in states such as septic shock and hypophosphataemia.Temperature
Temperature does not have such a dramatic effect compared to the previous factors, but hyperthermia causes a rightward shift, while hypothermia causes a leftward shift.Carbon monoxide
Haemoglobin binds with carbon monoxideCarbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
200-250 times more readily than with oxygen. The presence of carbon monoxide on one of the 4 haem
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
sites causes the oxygen on the other haem sites to bind with greater affinity. This makes it difficult for the haemoglobin to release oxygen to the tissues and has the effect of shifting the curve to the left (as well as downward, due to direct competitive effects of carbon monoxide). With an increased level of carbon monoxide, a person can suffer from severe tissue hypoxia while maintaining a normal pO2.
Effects of methaemoglobinaemia
MethaemoglobinaemiaMethemoglobinemia
Methemoglobinemia is a disorder characterized by the presence of a higher than normal level of methemoglobin in the blood. Methemoglobin is an oxidized form of hemoglobin that has an increased affinity for oxygen, resulting in a reduced ability to release oxygen to tissues. The oxygen–hemoglobin...
is a form of abnormal haemoglobin where ferrous (Fe2+), which is normally found in haemoglobin, is converted to the ferric (Fe3+) state. This causes a leftward shift in the curve as methaemoglobin does not unload O2 from Hb. However, methaemoglobin has increased affinity for cyanide, and is therefore useful in the treatment of cyanide poisoning.
Fetal haemoglobin
Fetal haemoglobin (HbF) is structurally different from normal adult haemoglobin (HbA). HbF is made up of two gamma and two alpha chains while adult hemoglobin, HbA, is made of two alpha and two beta chains. The fetal dissociation curve is shifted to the left relative to the curve for the normal adult because of these structural differences. Typically, fetal arterial oxygen pressures are lower than adult arterial oxygen pressures. Hence higher affinity to bind oxygen is required at lower levels of partial pressure in the fetus to allow diffusion of oxygen across the placentaPlacenta
The placenta is an organ that connects the developing fetus to the uterine wall to allow nutrient uptake, waste elimination, and gas exchange via the mother's blood supply. "True" placentas are a defining characteristic of eutherian or "placental" mammals, but are also found in some snakes and...
. At the placenta there is a higher concentration of 2,3-DPG formed which binds more readily to adult haemoglobin and not to fetal haemoglobin. This causes the adult HbA to release more oxygen at the placenta to be taken up by the fetus and put onto HbF. 2,3-DPG binds readily to beta chains and does not readily bind to gamma chains, hence HbF is not affected by 2,3-DPG. This exemplifies why the curve needs to be shifted to the left in the fetus. Oxygen unloading needs to be easier and oxygen binding needs to be improved at the lower pressures of fetal systemic circulation (a left shit) to provide oxygen to the developing fetus. HbA can pass oxygen on from maternal circulation across the placenta to the fetal circulation because HbF has a higher affinity to bind at lower partial pressures. HbF then delivers that bound oxygen to tissues that have even lower partial pressures where it can be released.


