Michaelis-Arbuzov reaction
Encyclopedia
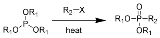
The Michaelis–Arbuzov reaction (also called the Arbuzov reaction) is the chemical reaction
of a trialkyl phosphite
and an alkyl halide to form a phosphonate
.
 The reaction was discovered by August Michaelis in 1898, and greatly explored by Aleksandr Arbuzov
The reaction was discovered by August Michaelis in 1898, and greatly explored by Aleksandr Arbuzov
soon thereafter. This reaction is widely used for the synthesis of various phosphonates, phosphinate
s, and phosphine oxide
s. Several reviews have been published.
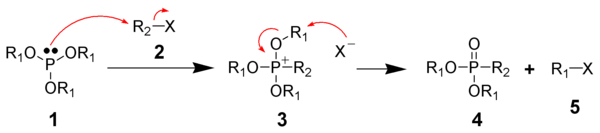 The Michaelis–Arbuzov reaction is initiated with the SN2 reaction
The Michaelis–Arbuzov reaction is initiated with the SN2 reaction
of the nucleophilic phosphite (1) with the electrophilic alkyl halide (2) to give a phosphonium intermediate (3). Triaryl phosphites, which are unable to perform the second step of the Michaelis-Arbuzov reaction, have been shown to produce stable phosphonium salts. Likewise, aryl
and vinyl
halides are less reactive towards phosphites.
The displaced halide
anion reacts via another SN2 reaction with the phosphonium intermediate to give the desired phosphonate (4) and another alkyl halide (5). When chiral phosphonium intermediates are produced, it has been shown the halide substitution proceeds with inversion of configuration, as expected by a SN2 reaction.
As a general guideline, the reactivity of the organic halide (2) can be listed as follows: (from most reactive to least reactive)
and
The reaction of α-bromo- and α-chloroketones with phosphites yields a vinyl phosphate instead of an alkyl phosphonate – the Perkow reaction
. α-Iodoketones do, in fact, give the a phosphonate. Other methods of producing β-ketophosphonates have been developed.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
of a trialkyl phosphite
Phosphite
A phosphite is a salt of phosphorous acid. The phosphite ion is a polyatomic ion with a phosphorus central atom where phosphorus has an oxidation state of +3...
and an alkyl halide to form a phosphonate
Phosphonate
Phosphonates or phosphonic acids are organic compounds containing C-PO2 or C-PO2 groups . Bisphosphonates were first synthesized in 1897 by Von Baeyer and Hofmann. An example of such a bisphosphonate is HEDP . Since the work of Schwarzenbach in 1949, phosphonic acids are known as effective...
.

Aleksandr Arbuzov
Aleksandr Erminingeldovich Arbuzov was a Russian/Soviet chemist who discovered the Michaelis–Arbuzov reaction.A native of Bilyarsk, Arbuzov studied in the Kazan University under Alexander Mikhaylovich Zaytsev. He graduated in 1900 and became professor at the same university in 1911...
soon thereafter. This reaction is widely used for the synthesis of various phosphonates, phosphinate
Phosphinate
Phosphinates are organophosphorus compounds with the formula OPR2.-See also:*Phosphine - PR3*Phosphine oxide - OPR3*Phosphinite - PR2*Phosphonite - P2R*Phosphite - P3*Phosphonate - OP2R*Phosphate - OP3...
s, and phosphine oxide
Phosphine oxide
Phosphine oxides are either inorganic phosphorus compounds such as phosphoryl trichloride or organophosphorus compounds with the formula OPR3, where R = alkyl or aryl...
s. Several reviews have been published.
Reaction mechanism

SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
of the nucleophilic phosphite (1) with the electrophilic alkyl halide (2) to give a phosphonium intermediate (3). Triaryl phosphites, which are unable to perform the second step of the Michaelis-Arbuzov reaction, have been shown to produce stable phosphonium salts. Likewise, aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
and vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
halides are less reactive towards phosphites.
The displaced halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
anion reacts via another SN2 reaction with the phosphonium intermediate to give the desired phosphonate (4) and another alkyl halide (5). When chiral phosphonium intermediates are produced, it has been shown the halide substitution proceeds with inversion of configuration, as expected by a SN2 reaction.
As a general guideline, the reactivity of the organic halide (2) can be listed as follows: (from most reactive to least reactive)
- RCOX > RCH2X > RR'CHX >> RR'R"CX
and
- RI > RBr > RCl
The reaction of α-bromo- and α-chloroketones with phosphites yields a vinyl phosphate instead of an alkyl phosphonate – the Perkow reaction
Perkow reaction
The Perkow reaction is an organic reaction in which a trialkyl phosphite ester reacts with a haloketone to form a dialkyl vinyl phosphate and an alkyl halide....
. α-Iodoketones do, in fact, give the a phosphonate. Other methods of producing β-ketophosphonates have been developed.
External links
- Ford-Moore, A. H.; Perry, B. J. Organic SynthesesOrganic SynthesesOrganic Syntheses is a scientific journal that since 1921 has provided the chemistry community with annual collections of detailed and checked procedures for the organic synthesis of organic compounds. The journal is peer reviewed...
, Coll. Vol. 4, p.325 (1963); Vol. 31, p.33 (1951). (Article) - Davidsen, S. K.; Phllips, G. W.; Martin, S. F. Organic SynthesesOrganic SynthesesOrganic Syntheses is a scientific journal that since 1921 has provided the chemistry community with annual collections of detailed and checked procedures for the organic synthesis of organic compounds. The journal is peer reviewed...
, Coll. Vol. 8, p.451 (1993); Vol. 65, p.119 (1987). (Article) - Enders, D.; von Berg, S.; Jandeleit, B. Organic SynthesesOrganic SynthesesOrganic Syntheses is a scientific journal that since 1921 has provided the chemistry community with annual collections of detailed and checked procedures for the organic synthesis of organic compounds. The journal is peer reviewed...
, Coll. Vol. 10, p.289 (2004); Vol. 78, p.169 (2002). (Article)

