_bromide.gif)
Gold(III) bromide
Encyclopedia
Gold bromide is a dark-red to black crystalline solid. It has the empirical formula
AuBr3, but exists primarily as a dimer with the molecular formula Au2Br6 in which two gold atoms are bridged by two bromine atoms. It is commonly referred to as gold(III) bromide, gold tribromide, and rarely but traditionally auric bromide, and sometimes as digold hexabromide. As is similar with the other gold halides, this compound is unique for being a coordination complex of a group 11 transition metal that is stable in an oxidation state
of three whereas copper or silver complexes persist in oxidation states of one or two.
. The gold centers exhibit square planar
coordination with bond angles of roughly 90 degrees.
Calculations indicate that in the hypothetical monomeric forms of the gold trihalides, the Jahn-Teller effect
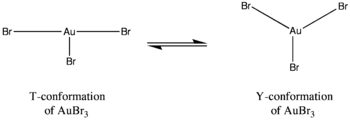
causes differences to arise in the structures of the gold halide complexes. For instance, gold(III) bromide contains one long and two short gold-bromine bonds whereas gold(III) chloride and gold(III) fluoride consist of two long and one short gold-halogen bonds. Moreover, gold tribromide does not exhibit the same coordination around the central gold atom as gold trichloride or gold trifluoride. In the latter complexes, the coordination exhibits a T-conformation, but in gold tribromide the coordination exists as more of a dynamic balance between a Y-conformation and a T-conformation. This coordination difference can be attributed to the Jahn-Teller effect
but more so to the decrease in π-back bonding of the gold atoms with the bromine ligands compared to the π-back bonding found with fluorine and chlorine ligands. It is also this decrease in π-back bonding which explains why gold tribromide is less stable than its trifluoride and trichloride counterparts.
at 140 °C:
Alternatively, the halide-exchange reaction of gold(III) chloride
with hydrobromic acid
has also been proven successful in synthesizing gold(III) bromide:
This reaction is driven by the production of the relatively more stable hydrochloric acid
compared with hydrobromic acid
.
three is not favored. Predominantly, gold(III) displays square planar coordination corresponding to a preferred coordination number of four.
Specifically, in solution gold(III) trihalides have the tendency to add a fourth ligand to form the more preferred four-coordinate complex. With respect to gold tribromide, it is common to purchase gold(III) bromide hydrate, AuBr3⋅H2O, where the central gold atom exhibits a coordination number of four, rather than the anhydrous
form of the compound, which exhibits a coordination number of three.
Alternatively, if there is no addition of a fourth ligand, gold tribromide will oligomerize to form the halogen-bridged dimer complex mentioned previously.
Furthermore, like gold(III) chloride
, gold tribromide is a Lewis acid and can form several complexes. For example, in the presence of hydrobromic acid
, the dimer dissolves and bromoauric acid is formed.
The dimer also undergoes hydrolysis
rapidly in moist air.
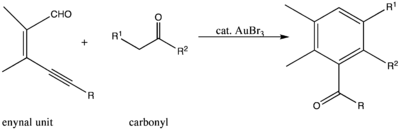
. Specifically, the compound catalyzes the reaction between an enynal unit and carbonyl
compounds to form a six-membered cyclic compound.
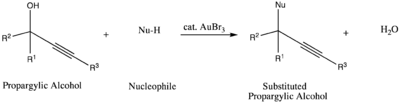
Another catalytic use of gold tribromide is in the nucleophilic substitution
reaction of propargylic alcohols. In this reaction, the gold complex acts as an alcohol-activating agent to facilitate the substitution.
Empirical formula
In chemistry, the empirical formula of a chemical compound is the simplest positive integer ratio of atoms of each element present in a compound. An empirical formula makes no reference to isomerism, structure, or absolute number of atoms. The empirical formula is used as standard for most ionic...
AuBr3, but exists primarily as a dimer with the molecular formula Au2Br6 in which two gold atoms are bridged by two bromine atoms. It is commonly referred to as gold(III) bromide, gold tribromide, and rarely but traditionally auric bromide, and sometimes as digold hexabromide. As is similar with the other gold halides, this compound is unique for being a coordination complex of a group 11 transition metal that is stable in an oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
of three whereas copper or silver complexes persist in oxidation states of one or two.
History
The first mention of any research or study of the gold halides dates back to the early-to-mid-19th century, and there are three primary researchers associated with the extensive investigation of this particular area of chemistry: Thomsen, Schottländer, and Krüss.Structure
The dimer, digold hexabromide, has structural properties similar to those of the other gold trihalide dimeric compounds, such as gold(III) chlorideGold(III) chloride
Gold chloride, traditionally called auric chloride, is a chemical compound of gold and chlorine. With the molecular formula Au2Cl6, the name gold trichloride is a simplification, referring to the empirical formula. The Roman numerals in the name indicate that the gold has an oxidation state of +3,...
. The gold centers exhibit square planar
Square planar
The square planar molecular geometry in chemistry describes the stereochemistry that is adopted by certain chemical compounds...
coordination with bond angles of roughly 90 degrees.
Calculations indicate that in the hypothetical monomeric forms of the gold trihalides, the Jahn-Teller effect
Jahn-Teller effect
The Jahn–Teller effect, sometimes also known as Jahn–Teller distortion, or the Jahn–Teller theorem, describes the geometrical distortion of non-linear molecules under certain situations. This electronic effect is named after Hermann Arthur Jahn and Edward Teller, who proved, using group theory,...
causes differences to arise in the structures of the gold halide complexes. For instance, gold(III) bromide contains one long and two short gold-bromine bonds whereas gold(III) chloride and gold(III) fluoride consist of two long and one short gold-halogen bonds. Moreover, gold tribromide does not exhibit the same coordination around the central gold atom as gold trichloride or gold trifluoride. In the latter complexes, the coordination exhibits a T-conformation, but in gold tribromide the coordination exists as more of a dynamic balance between a Y-conformation and a T-conformation. This coordination difference can be attributed to the Jahn-Teller effect
Jahn-Teller effect
The Jahn–Teller effect, sometimes also known as Jahn–Teller distortion, or the Jahn–Teller theorem, describes the geometrical distortion of non-linear molecules under certain situations. This electronic effect is named after Hermann Arthur Jahn and Edward Teller, who proved, using group theory,...
but more so to the decrease in π-back bonding of the gold atoms with the bromine ligands compared to the π-back bonding found with fluorine and chlorine ligands. It is also this decrease in π-back bonding which explains why gold tribromide is less stable than its trifluoride and trichloride counterparts.
Preparation
The most common synthesis method of gold(III) bromide is heating gold and excess liquid bromineBromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
at 140 °C:
- 2 Au + 3 Br2 → Au2Br6
Alternatively, the halide-exchange reaction of gold(III) chloride
Gold(III) chloride
Gold chloride, traditionally called auric chloride, is a chemical compound of gold and chlorine. With the molecular formula Au2Cl6, the name gold trichloride is a simplification, referring to the empirical formula. The Roman numerals in the name indicate that the gold has an oxidation state of +3,...
with hydrobromic acid
Hydrobromic acid
Hydrobromic acid is a strong acid formed by dissolving the diatomic molecule hydrogen bromide in water. "Constant boiling" hydrobromic acid is an aqueous solution that distills at 124.3 °C and contains 47.6% HBr by weight, which is 8.89 mol/L. Hydrobromic acid has a pKa of −9, making it a...
has also been proven successful in synthesizing gold(III) bromide:
- Au2Cl6 + 6 HBr → 6 HCl + Au2Br6
This reaction is driven by the production of the relatively more stable hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
compared with hydrobromic acid
Hydrobromic acid
Hydrobromic acid is a strong acid formed by dissolving the diatomic molecule hydrogen bromide in water. "Constant boiling" hydrobromic acid is an aqueous solution that distills at 124.3 °C and contains 47.6% HBr by weight, which is 8.89 mol/L. Hydrobromic acid has a pKa of −9, making it a...
.
Chemical Properties
The neutral monomer AuBr3, as well as the other neutral gold trihalide species, has not been isolated in the gas phase which indicates the coordination numberCoordination number
In chemistry and crystallography, the coordination number of a central atom in a molecule or crystal is the number of its nearest neighbours. This number is determined somewhat differently for molecules and for crystals....
three is not favored. Predominantly, gold(III) displays square planar coordination corresponding to a preferred coordination number of four.
Specifically, in solution gold(III) trihalides have the tendency to add a fourth ligand to form the more preferred four-coordinate complex. With respect to gold tribromide, it is common to purchase gold(III) bromide hydrate, AuBr3⋅H2O, where the central gold atom exhibits a coordination number of four, rather than the anhydrous
Anhydrous
As a general term, a substance is said to be anhydrous if it contains no water. The way of achieving the anhydrous form differs from one substance to another...
form of the compound, which exhibits a coordination number of three.
Alternatively, if there is no addition of a fourth ligand, gold tribromide will oligomerize to form the halogen-bridged dimer complex mentioned previously.
- 2 AuBr3 → Au2Br6
Furthermore, like gold(III) chloride
Gold(III) chloride
Gold chloride, traditionally called auric chloride, is a chemical compound of gold and chlorine. With the molecular formula Au2Cl6, the name gold trichloride is a simplification, referring to the empirical formula. The Roman numerals in the name indicate that the gold has an oxidation state of +3,...
, gold tribromide is a Lewis acid and can form several complexes. For example, in the presence of hydrobromic acid
Hydrobromic acid
Hydrobromic acid is a strong acid formed by dissolving the diatomic molecule hydrogen bromide in water. "Constant boiling" hydrobromic acid is an aqueous solution that distills at 124.3 °C and contains 47.6% HBr by weight, which is 8.89 mol/L. Hydrobromic acid has a pKa of −9, making it a...
, the dimer dissolves and bromoauric acid is formed.
- HBr (aq) + AuBr3 (aq) → H+AuBr4− (aq)
The dimer also undergoes hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
rapidly in moist air.
Uses
Gold(III) bromide is used as a catalyst in a variety of reactions, but one of its most interesting uses is found in the Diels-Alder reactionDiels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
. Specifically, the compound catalyzes the reaction between an enynal unit and carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
compounds to form a six-membered cyclic compound.
Another catalytic use of gold tribromide is in the nucleophilic substitution
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
reaction of propargylic alcohols. In this reaction, the gold complex acts as an alcohol-activating agent to facilitate the substitution.
External links
- http://www.sigmaaldrich.com/catalog/search/ProductDetail/ALDRICH/398470 Sigma Alrich Product info for Gold(III) Bromide
- http://www.webelements.com/webelements/compounds/text/Au/Au2Br6-10294287.html Web Elements info page for Gold(III) Bromide