
Glycerophospholipid
Encyclopedia

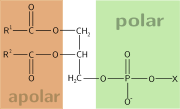
Glycerophospholipids or phosphoglycerides are glycerol
Glycerol
Glycerol is a simple polyol compound. It is a colorless, odorless, viscous liquid that is widely used in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature. The glycerol backbone is central to all lipids...
-based phospholipid
Phospholipid
Phospholipids are a class of lipids that are a major component of all cell membranes as they can form lipid bilayers. Most phospholipids contain a diglyceride, a phosphate group, and a simple organic molecule such as choline; one exception to this rule is sphingomyelin, which is derived from...
s. They are the main component of biological membrane
Biological membrane
A biological membrane or biomembrane is an enclosing or separatingmembrane that acts as a selective barrier, within or around a cell. It consists of a lipid bilayer with embedded proteins that may constitute close to 50% of membrane content...
s.
Structures
The term glycerophospholipid signifies any derivative of sn-glycero-3-phosphoric acid that contains at least one O-acylAcyl
An acyl group is a functional group derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids.In organic chemistry, the acyl group is usually derived from a carboxylic acid . Therefore, it has the formula RCO-, where R represents an alkyl group that is...
, or O-alkyl, or O-alk-1'-enyl residue
Residue (chemistry)
In chemistry, residue is the material remaining after a distillation or an evaporation, or to a portion of a larger molecule, such as a methyl group. It may also refer to the undesired byproducts of a reaction....
attached to the glycerol moiety
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
and a polar
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
head made of a nitrogenous base, a glycerol or an inositol
Inositol
Inositol or cyclohexane-1,2,3,4,5,6-hexol is a chemical compound with formula 6126 or 6, a sixfold alcohol of cyclohexane. It exists in nine possible stereoisomers, of which the most prominent form, widely occurring in nature, is cis-1,2,3,5-trans-4,6-cyclohexanehexol, or myo-inositol...
unit.
The alcohol here is glycerol, to which two fatty acids and a phosphoric acid are attached as esters. This basic structure is a phosphatidate. Phosphatidate is an important intermediate in the synthesis of many phosphoglycerides. The presence of an additional group attached to the phosphate allows for many different phosphoglycerides.
By convention, structures of these compounds show the 3 glycerol carbon attoms vertically with the phosphate attached to carbon atom number three (at the bottom). The occurrence of phosphoglycerides is almost exclusive to plant and animal cell membranes. Plasmalogens and phosphatidates are examples.
Nomenclature and stereochemistry
In general, glycerophospholipids use a "sn" notation, which stands for stereochemicalStereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
numbering. When the letters "sn" appear in the nomenclature, by convention the hydroxyl group of the second carbon of glycerol (sn-2) is on the left on a Fischer projection
Fischer projection
The Fischer projection, devised by Hermann Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates and used by chemists, particularly in organic chemistry and...
. The numbering follows the one of Fischer's projections, being sn-1 the carbon at the top and sn-3 the one at the bottom.
The advantage of this particular notation is that the spatial conformation (R or L) of the glycero-molecule is determined intuitively by the residues on the positions sn-1 and sn-3.
For example sn-glycero-3-phosphoric acid
Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
and sn-glycero-1-phosphoric acid are enantiomers.
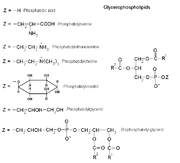
Examples of glycerophospholipids
PlasmalogensPlasmalogens are a type of phosphoglyceride. The first carbon of glycerol has a hydrocarbon chain attached via an ether, not ester, linkage. Ther linkages are more resistant to chemical attack than ester linkages are. The second (central) carbon atom has a fatty acid linked by an ester. The third carbon links to an ethanolamine or choline by means of a phosphate ester. These compounds are key components of the membranes of muscles and nerves.
Phosphatidates
Phosphatidates are lipids in which the first two carbon atoms of the glycerol are fatty acid esters, and the 3 is a phosphate ester. The phosphate serves as a link to another alchol-usually ethanolamine, choline, serine, or a carbohydrate. The identity of the alchol determines the subcategory of the phosphatidate. There is a negative charge on the phosphate and, in the case of choline or serine, a positive quaternary ammonium ion. (Serine also has a negative carboxylate group.) the presence of charges give a "head" with an overall charge. The phosphate ester portion ("head") is hydrophillic, whereas the remainder of the molecule, the fatty acid "tail", is hydrophobic. These are important components for the formation of lipid bilayers.
Phosphatidylethanoamines, phosphatidylcholines, and other phospholipids are examples of phosphatidates.
Phosphatidylcholines
These are the lecithins. Choline is the alcohol, with a positively charged quaternary ammonium, bound to the phosphate, with a negative charge. Lecithins are present in all living organisms. An egg yolk has a high concentration of lecthins- which are commercially important as an emulsifying agent in products such as mayonnaise. Lechithins are also present in brain and nerve tissue.
Other phospholipids
There are many other phospholipids, some of which are glycolipids. The glycolipids include phosphatidyl sugars where the alchol functional group is part of a carbohydrate. Phosphatidyl sugars are present in plants and certain microorganisms. A carbohydrate is very hydrophillic due to the large number of hydroxyl groups present.
Use in membranes
One of a glycerophospholipid's functions is to serve as a structural component of cell membraneCell membrane
The cell membrane or plasma membrane is a biological membrane that separates the interior of all cells from the outside environment. The cell membrane is selectively permeable to ions and organic molecules and controls the movement of substances in and out of cells. It basically protects the cell...
s. The cell membrane seen under the electron microscope
Electron microscope
An electron microscope is a type of microscope that uses a beam of electrons to illuminate the specimen and produce a magnified image. Electron microscopes have a greater resolving power than a light-powered optical microscope, because electrons have wavelengths about 100,000 times shorter than...
consists of two identifiable layers, or "leaflets", each of which is made up of an ordered row of glycerophospholipid molecules. The composition of each layer can vary widely depending on the type of cell.
- For example, in human erythrocytes the cytosolic side (the side facing the cytosolCytosolThe cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments....
) of the plasma membrane consists mainly of phosphatidylethanolaminePhosphatidylethanolaminePhosphatidylethanolamine is a lipid found in biological membranes. It is synthesized by the addition of CDP-ethanolamine to diglyceride, releasing CMP. S-adenosyl methionine can subsequently methylate the amine of phosphatidyl ethanolamine to yield phosphatidyl choline.Cephalin is a phospholipid,...
, phosphatidylserinePhosphatidylserinePhosphatidylserine is a phospholipid component, usually kept on the inner-leaflet of cell membranes by an enzyme called flippase...
, and phosphatidylinositol. - By contrast, the exoplasmic side (the side on the exterior of the cell) consists mainly of phosphatidylcholinePhosphatidylcholinePhosphatidylcholines are a class of phospholipids that incorporate choline as a headgroup.They are a major component of biological membranes and can be easily obtained from a variety of readily available sources such as egg yolk or soy beans from which they are mechanically extracted or chemically...
and sphingomyelinSphingomyelinSphingomyelin is a type of sphingolipid found in animal cell membranes, especially in the membranous myelin sheath that surrounds some nerve cell axons. It usually consists of phosphorylcholine and ceramide...
, a type of sphingolipidSphingolipidSphingolipids are a class of lipids containing a backbone of sphingoid bases, a set of aliphatic amino alcohols that includes sphingosine. They were discovered in brain extracts in the 1870s and were named for the mythological Sphinx because of their enigmatic nature. These compounds play...
.
Each glycerophospholipid molecule consists of a small polar
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
head group and two long hydrophobic chains. In the cell membrane, the two layers of phospholipids are arranged as follows:
- the hydrophobic tails point to each other and form a fatty, hydrophobic center
- the ionicIonic compoundIn chemistry, an ionic compound is a chemical compound in which ions are held together in a lattice structure by ionic bonds. Usually, the positively charged portion consists of metal cations and the negatively charged portion is an anion or polyatomic ion. Ions in ionic compounds are held together...
head groups are placed at the inner and outer surfaces of the cell membrane
This is a stable structure because the ionic hydrophilic head groups interact with the aqueous media inside and outside the cell, whereas the hydrophobic tails maximize hydrophobic interactions with each other and are kept away from the aqueous environments. The overall result of this structure is to construct a fatty barrier between the cell's interior and its surroundings.
Use in emulsification
Glycerophospholipids can also act as an emulsifying agent to promote dispersal of one substance into another. This is sometimes used in candyCandy
Candy, specifically sugar candy, is a confection made from a concentrated solution of sugar in water, to which flavorings and colorants are added...
making and ice-cream making.
Glycerolphospholipids in the brain
Neural membranes contain several classes of glycerophospholipids which turnover at different rates with respect to their structure and localization in different cells and membranes. The glycerophospholipid composition of neural membranes greatly alters their functional efficacy. The length of glycerophospholipid acyl chain and the degree of saturation are important determinants of many membrane characteristics including the formation of lateral domains that are rich in polyunsaturated fatty acids. Receptor-mediated degradation of glycerophospholipids by phospholipases A(l), A(2), C, and D results in generation of second messengers such as arachidonic acidArachidonic acid
Arachidonic acid is a polyunsaturated omega-6 fatty acid 20:4.It is the counterpart to the saturated arachidic acid found in peanut oil, Arachidonic acid (AA, sometimes ARA) is a polyunsaturated omega-6 fatty acid 20:4(ω-6).It is the counterpart to the saturated arachidic acid found in peanut oil,...
, eicosanoids, platelet activating factor and diacylglycerol. Thus, neural membrane phospholipids are a reservoir for second messengers. They are also involved in apoptosis, modulation of activities of transporters, and membrane-bound enzymes. Marked alterations in neural membrane glycerophospholipid composition have been reported to occur in neurological disorders. These alterations result in changes in membrane fluidity and permeability. These processes along with the accumulation of lipid peroxides and compromised energy metabolism may be responsible for the neurodegeneration observed in neurological disorders.

