
Gas phase ion chemistry
Encyclopedia
Gas phase ion chemistry is a field of science encompassed within both chemistry
and physics
. It is the science that studies ion
s and molecule
s in the gas phase, most often enabled by some form of mass spectrometry
. By far the most important applications for this science is in studying the thermodynamics and kinetics
of reactions. For example one application is in studying the thermodynamics
of the solvation
of ions. Ions with small solvation
spheres of 1, 2, 3... solvent molecules can be studied in the gas phase and then extrapolated to bulk solution.
(quasi-equilibrium) between reactants and activated complexes.
reaction rates from a few characteristics of the potential energy surface
.
or molecule
into an ion
by adding or removing charged particles such as electron
s or other ions can occur in the gas phase. These processes are an important component of gas phase ion chemistry.
s or molecule
s interact to form a single product ion
.
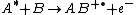
where species A with excess internal energy (indicated by the asterisk) interacts with B to form the ion AB+.
One or both of the interacting species may have excess internal energy
.
and a neutral species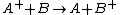
in which the charge of the ion is transferred to the neutral.
, ammonia
, and isobutane
.
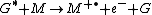
where G is the excited state species (indicated by the superscripted asterisk), and M is the species that is ionized by the loss of an electron
to form the radical
cation (indicated by the superscripted "plus-dot").
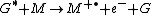
Penning ionization occurs when the target molecule has an ionization potential
lower than the internal energy of the excited-state atom or molecule. Associative Penning ionization can also occur:
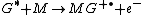
reactions that take place in the gas phase.
s in the gas phase. The molecular ions collide with neutral gas molecules such as helium
, nitrogen
or argon
. In the collision some of the kinetic energy is converted into internal energy
which results in fragmentation.
breaking that occurs in a gas phase ion
in which the cleaved bond is not adjacent to the location of the charge.
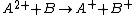 ,
,
charge-stripping reaction
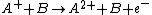 ,
,
and charge-inversion reaction positive to negative
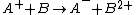
and negative to positive
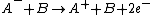 .
.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
and physics
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
. It is the science that studies ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s and molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s in the gas phase, most often enabled by some form of mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
. By far the most important applications for this science is in studying the thermodynamics and kinetics
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
of reactions. For example one application is in studying the thermodynamics
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
of the solvation
Solvation
Solvation, also sometimes called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute...
of ions. Ions with small solvation
Solvation
Solvation, also sometimes called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute...
spheres of 1, 2, 3... solvent molecules can be studied in the gas phase and then extrapolated to bulk solution.
Transition state theory
Transition state theory is the theory of the rates of elementary reactions which assumes a special type of chemical equilibriumChemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
(quasi-equilibrium) between reactants and activated complexes.
RRKM theory
RRKM theory is used to compute simple estimates of the unimolecular ion decompositionUnimolecular ion decomposition
Unimolecular ion decomposition is the fragmentation of a gas phase ion in a reaction with a molecularity of one. Ions with sufficient internal energy may fragment in a mass spectrometer, which in some cases may degrade the mass spectrometer performance, but in other cases, such as tandem mass...
reaction rates from a few characteristics of the potential energy surface
Potential energy surface
A potential energy surface is generally used within the adiabatic or Born–Oppenheimer approximation in quantum mechanics and statistical mechanics to model chemical reactions and interactions in simple chemical and physical systems...
.
Gas phase ion formation
The process of converting an atomAtom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
or molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
into an ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
by adding or removing charged particles such as electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s or other ions can occur in the gas phase. These processes are an important component of gas phase ion chemistry.
Associative ionization
Associative ionization is a gas phase reaction in which two atomAtom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s or molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s interact to form a single product ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
.
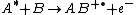
where species A with excess internal energy (indicated by the asterisk) interacts with B to form the ion AB+.
One or both of the interacting species may have excess internal energy
Internal energy
In thermodynamics, the internal energy is the total energy contained by a thermodynamic system. It is the energy needed to create the system, but excludes the energy to displace the system's surroundings, any energy associated with a move as a whole, or due to external force fields. Internal...
.
Charge-exchange ionization
Charge-exchange ionization (also called charge-transfer ionization) is a gas phase reaction between an ionIon
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
and a neutral species
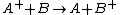
in which the charge of the ion is transferred to the neutral.
Chemical ionization
In chemical ionization, ions are produced through the reaction of ions of a reagent gas with other species. Some common reagent gases include: methaneMethane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
, ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
, and isobutane
Isobutane
Isobutane, also known as methylpropane, is an isomer of butane. It is the simplest alkane with a tertiary carbon. Concerns with depletion of the ozone layer by freon gases have led to increased use of isobutane as a gas for refrigeration systems, especially in domestic refrigerators and freezers,...
.
Chemi-ionization
Chemi-ionization can be represented by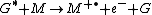
where G is the excited state species (indicated by the superscripted asterisk), and M is the species that is ionized by the loss of an electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
to form the radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
cation (indicated by the superscripted "plus-dot").
Penning ionization
Penning ionization refers to the interaction between a gas-phase excited-state atom or molecule G* and a target molecule M resulting in the formation of a radical molecular cation M+., an electron e−, and a neutral gas molecule G: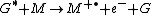
Penning ionization occurs when the target molecule has an ionization potential
Ionization potential
The ionization energy of a chemical species, i.e. an atom or molecule, is the energy required to remove an electron from the species to a practically infinite distance. Large atoms or molecules have a low ionization energy, while small molecules tend to have higher ionization energies.The property...
lower than the internal energy of the excited-state atom or molecule. Associative Penning ionization can also occur:
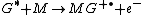
Fragmentation
There are many important dissociationDissociation (chemistry)
Dissociation in chemistry and biochemistry is a general process in which ionic compounds separate or split into smaller particles, ions, or radicals, usually in a reversible manner...
reactions that take place in the gas phase.
Collision-induced dissociation
CID (also called collisionally activated dissociation - CAD) is a method used to fragment molecular ionIon
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s in the gas phase. The molecular ions collide with neutral gas molecules such as helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
, nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
or argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
. In the collision some of the kinetic energy is converted into internal energy
Internal energy
In thermodynamics, the internal energy is the total energy contained by a thermodynamic system. It is the energy needed to create the system, but excludes the energy to displace the system's surroundings, any energy associated with a move as a whole, or due to external force fields. Internal...
which results in fragmentation.
Charge remote fragmentation
Charge remote fragmentation is a type of covalent bondCovalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
breaking that occurs in a gas phase ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
in which the cleaved bond is not adjacent to the location of the charge.
Charge transfer reactions
There are several types of charge-transfer reactions (also known as charge-permutation reactions): partial-charge transfer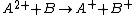 ,
,charge-stripping reaction
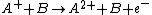 ,
,and charge-inversion reaction positive to negative
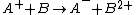
and negative to positive
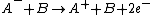 .
.See also
- Adiabatic ionizationAdiabatic ionizationAdiabatic ionization is a form of ionization in which an electron is removed from or added to an atom or molecule in its lowest energy state to form an ion in its lowest energy state....
- Mass-analyzed ion kinetic energy spectrometryMass-analyzed ion kinetic energy spectrometryMass-analyzed ion-kinetic-energy spectrometry is a mass spectrometry technique by which mass spectra are obtained from a sector instrument that incorporates at least one magnetic sector plus one electric sector in reverse geometry...
- Plasma (physics)Plasma (physics)In physics and chemistry, plasma is a state of matter similar to gas in which a certain portion of the particles are ionized. Heating a gas may ionize its molecules or atoms , thus turning it into a plasma, which contains charged particles: positive ions and negative electrons or ions...
- Michael T. BowersMichael T. BowersMichael T. Bowers is a professor in the Department of Chemistry and Biochemistry at UC Santa Barbara and a Fellow of the American Chemical Society .- Early life and education :* 1962 B.S. Gonzaga University* 1966 Ph.D. University of Illinois Michael T. Bowers is a professor in the Department of...
- R. Graham CooksR. Graham Cooks- Early life and education :* 1961 B.S. University of Natal, South Africa* 1965 Ph.D. University of Natal, South Africa* 1967 Ph.D. Cambridge University, Great Britain- Research interests :...

