
Dithioerythritol
Encyclopedia
Dithioerythritol is a sulfur containing sugar derived from the corresponding 4-carbon monosaccharide
erythrose
. It is an epimer
of dithiothreitol
(DTT). The molecular formula for DTE is C4H10O2S2.
Like DTT, DTE makes an excellent reducing agent
, although its standard reduction potential
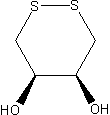
is not quite as negative, i.e., DTE is slightly less effective at reducing than DTT, presumably because steric repulsion of its OH groups makes the cyclic disulfide-bonded form
of DTE less favorable. In DTT, these hydroxyl groups
are on opposite sides of the ring, whereas they are on the same side of the ring (and, hence, closer) in DTE.
Monosaccharide
Monosaccharides are the most basic units of biologically important carbohydrates. They are the simplest form of sugar and are usually colorless, water-soluble, crystalline solids. Some monosaccharides have a sweet taste. Examples of monosaccharides include glucose , fructose , galactose, xylose...
erythrose
Erythrose
Erythrose is a tetrose carbohydrate with chemical formula C4H8O4. It has one aldehyde group and so is part of the aldose family. The natural isomer is D-erythrose....
. It is an epimer
Epimer
In chemistry, epimers are diastereomers that differ in configuration of only one stereogenic center. Diastereomers are a class of stereoisomers that are non-superposable, non-mirror images of one another....
of dithiothreitol
Dithiothreitol
Dithiothreitol is the common name for a small-molecule redox reagent known as Cleland's reagent. DTT's formula is C4H10O2S2 and the molecular structure of its reduced form is shown at the right; its oxidized form is a disulfide-bonded 6-membered ring . Its name derives from the four-carbon...
(DTT). The molecular formula for DTE is C4H10O2S2.
Like DTT, DTE makes an excellent reducing agent
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
, although its standard reduction potential
Reduction potential
Reduction potential is a measure of the tendency of a chemical species to acquire electrons and thereby be reduced. Reduction potential is measured in volts , or millivolts...
is not quite as negative, i.e., DTE is slightly less effective at reducing than DTT, presumably because steric repulsion of its OH groups makes the cyclic disulfide-bonded form
Disulfide bond
In chemistry, a disulfide bond is a covalent bond, usually derived by the coupling of two thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry...
of DTE less favorable. In DTT, these hydroxyl groups
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
are on opposite sides of the ring, whereas they are on the same side of the ring (and, hence, closer) in DTE.


