
Dithiothreitol
Encyclopedia
Dithiothreitol is the common name for a small-molecule redox
reagent known as Cleland's
reagent. DTT's formula is C4H10O2S2 and the molecular structure of its reduced form is shown at the right; its oxidized form is a disulfide-bonded
6-membered ring (shown below). Its name derives from the four-carbon sugar
, threose
. DTT has an epimer
ic ('sister') compound, dithioerythritol
(DTE).
, owing to its high conformational propensity to form a six-membered ring with an internal disulfide bond
. It has a redox potential of -0.33 V at pH 7. The reduction of a typical disulfide bond proceeds by two sequential thiol-disulfide exchange reactions and is illustrated below. The intermediate mixed-disulfide state is unstable (i.e., poorly populated) because the second thiol of DTT has a high propensity to close the ring, forming oxidized DTT and leaving behind a reduced disulfide bond
. The reducing power of DTT is limited to pH values above ~7, since only the negatively charged thiolate form -S– is reactive (the protonated thiol
form -SH is not); the pKa
of the thiol groups is 9.2 and 10.1.
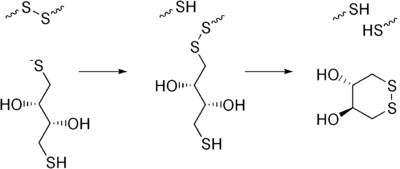
have a tendency to form dimers in solution, especially in the presence of oxygen. Dimerization greatly lowers the efficiency of subsequent coupling reactions such as DNA immobilization on gold in biosensors. Typically DTT is mixed with a DNA solution and allowed to react, and then is removed by filtration (for the solid catalyst) or by chromatography
(for the liquid form). The DTT removal procedure is often called "desalting."
DTT is frequently used to reduce the disulfide bond
s of protein
s and, more generally, to prevent intramolecular and intermolecular disulfide bonds from forming between cysteine
residues of proteins. However, even DTT cannot reduce buried (solvent-inaccessible) disulfide bonds, so reduction of disulfide bonds is sometimes carried out under denaturing conditions
(e.g., at high temperature
s, or in the presence of a strong denaturant such as 6 M guanidinium hydrochloride
, 8 M urea
, or 1% sodium dodecylsulfate). Conversely, the solvent exposure of different disulfide bonds can be assayed by their rate of reduction in the presence of DTT.
DTT can also be used as an oxidizing agent
. Its principal advantage is that effectively no mixed-disulfide species are populated, in contrast to other agents such as glutathione
. In very rare cases, a DTT adduct
may be formed, i.e., the two sulfur atoms of DTT may form disulfide bond
s to different sulfur atoms; in such cases, DTT cannot cyclize since it has no remaining free thiols.
hydrochloride) is an alternative which is more stable and works even at low pH.
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
reagent known as Cleland's
W. Wallace Cleland
William Wallace Cleland is a University of Wisconsin-Madison biochemistry professor. His research focuses on enzyme reaction mechanism and enzyme kinetics. He made significant contributions to our understanding of multiple substrate enzymes...
reagent. DTT's formula is C4H10O2S2 and the molecular structure of its reduced form is shown at the right; its oxidized form is a disulfide-bonded
Disulfide bond
In chemistry, a disulfide bond is a covalent bond, usually derived by the coupling of two thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry...
6-membered ring (shown below). Its name derives from the four-carbon sugar
Monosaccharide
Monosaccharides are the most basic units of biologically important carbohydrates. They are the simplest form of sugar and are usually colorless, water-soluble, crystalline solids. Some monosaccharides have a sweet taste. Examples of monosaccharides include glucose , fructose , galactose, xylose...
, threose
Threose
Threose is a four-carbon monosaccharide or carbohydrate with molecular formula C4H8O4. It has a terminal aldehyde group rather than a ketone in its linear chain, and so is considered part of the aldose family of monosaccharides...
. DTT has an epimer
Epimer
In chemistry, epimers are diastereomers that differ in configuration of only one stereogenic center. Diastereomers are a class of stereoisomers that are non-superposable, non-mirror images of one another....
ic ('sister') compound, dithioerythritol
Dithioerythritol
Dithioerythritol is a sulfur containing sugar derived from the corresponding 4-carbon monosaccharide erythrose. It is an epimer of dithiothreitol...
(DTE).
Reducing agent
DTT is an unusually strong reducing agentReducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
, owing to its high conformational propensity to form a six-membered ring with an internal disulfide bond
Disulfide bond
In chemistry, a disulfide bond is a covalent bond, usually derived by the coupling of two thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry...
. It has a redox potential of -0.33 V at pH 7. The reduction of a typical disulfide bond proceeds by two sequential thiol-disulfide exchange reactions and is illustrated below. The intermediate mixed-disulfide state is unstable (i.e., poorly populated) because the second thiol of DTT has a high propensity to close the ring, forming oxidized DTT and leaving behind a reduced disulfide bond
Disulfide bond
In chemistry, a disulfide bond is a covalent bond, usually derived by the coupling of two thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry...
. The reducing power of DTT is limited to pH values above ~7, since only the negatively charged thiolate form -S– is reactive (the protonated thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
form -SH is not); the pKa
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
of the thiol groups is 9.2 and 10.1.

Applications
A common use of DTT is as a reducing or "deprotecting" agent for thiolated DNA. The terminal sulfur atoms of thiolated DNADNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
have a tendency to form dimers in solution, especially in the presence of oxygen. Dimerization greatly lowers the efficiency of subsequent coupling reactions such as DNA immobilization on gold in biosensors. Typically DTT is mixed with a DNA solution and allowed to react, and then is removed by filtration (for the solid catalyst) or by chromatography
Chromatography
Chromatography is the collective term for a set of laboratory techniques for the separation of mixtures....
(for the liquid form). The DTT removal procedure is often called "desalting."
DTT is frequently used to reduce the disulfide bond
Disulfide bond
In chemistry, a disulfide bond is a covalent bond, usually derived by the coupling of two thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry...
s of protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s and, more generally, to prevent intramolecular and intermolecular disulfide bonds from forming between cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
residues of proteins. However, even DTT cannot reduce buried (solvent-inaccessible) disulfide bonds, so reduction of disulfide bonds is sometimes carried out under denaturing conditions
Denaturation (biochemistry)
Denaturation is a process in which proteins or nucleic acids lose their tertiary structure and secondary structure by application of some external stress or compound, such as a strong acid or base, a concentrated inorganic salt, an organic solvent , or heat...
(e.g., at high temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
s, or in the presence of a strong denaturant such as 6 M guanidinium hydrochloride
Guanidine
Guanidine is a crystalline compound of strong alkalinity formed by the oxidation of guanine. It is used in the manufacture of plastics and explosives. It is found in urine as a normal product of protein metabolism. The molecule was first synthesized in 1861 by the oxidative degradation of an...
, 8 M urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
, or 1% sodium dodecylsulfate). Conversely, the solvent exposure of different disulfide bonds can be assayed by their rate of reduction in the presence of DTT.
DTT can also be used as an oxidizing agent
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
. Its principal advantage is that effectively no mixed-disulfide species are populated, in contrast to other agents such as glutathione
Glutathione
Glutathione is a tripeptide that contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side-chain...
. In very rare cases, a DTT adduct
Adduct
An adduct is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is considered a distinct molecular species...
may be formed, i.e., the two sulfur atoms of DTT may form disulfide bond
Disulfide bond
In chemistry, a disulfide bond is a covalent bond, usually derived by the coupling of two thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry...
s to different sulfur atoms; in such cases, DTT cannot cyclize since it has no remaining free thiols.
Properties
Due to air oxidation, DTT is a relatively unstable compound whose useful life can be extended by refrigeration and handling in an inert atmosphere. Since protonated sulfurs have lowered nucleophilicities, DTT becomes less potent as the pH lowers. Tris(2-carboxyethyl)phosphine HCl (TCEPTCEP
TCEP is a reducing agent frequently used in biochemistry and molecular biology applications. It is often prepared and used as a hydrochloride salt with a molecular weight of 286.65 gram/mol...
hydrochloride) is an alternative which is more stable and works even at low pH.

