
Cannabinoid receptor antagonist
Encyclopedia
The discovery of the endogenous cannabinoid system led to the development of CB1 receptor
antagonists. The first cannabinoid receptor "antagonist," rimonabant
, was described in 1994. Rimonabant blocks the CB1 receptor selectively and it has been shown to decrease food intake and regulate body-weight gain. The prevalence of obesity
worldwide is increasing dramatically and has a great impact on public health. The lack of efficient and well-tolerated drugs to cure obesity has led to an increased interest in research and development of cannabinoid antagonists.
 For centuries hashish
For centuries hashish
and marijuana from the Indian hemp Cannabis sativa
L. have been used for medicinal and recreational purposes. In 1840, Schlesinger S. was apparently the first investigator to obtain an active extract from the leaves and flowers of hemp. A few years later, in 1848, Decourtive E. described the preparation of an ethanol extract that on evaporation of the solvent gave a dark resin, which he named “cannabin”.http://www.dialogues-cns.com/brochures/35/pdf/35.pdf#page=61. In 1964 the main active constituent of C. sativa L., Δ9-tetrahydrocannabinol (THC
), was isolated and synthesized by Mechoulam's
laboratory. Two types of cannabinoid receptors, CB1 and CB2, responsible for the effects of THC were discovered and cloned in the early 1990s. Once cannabinoid receptors had been discovered, it became important to establish whether their agonists occur naturally in the body. This search led to the discovery of the first endogenous cannabinoid (endocannabinoid), anandamide
(arachidonoyl ethanolamide). Later on other endocannabinoids were found, for example 2-AG (2-arachidonoyl glycerol). These findings raised further questions about the pharmacological and physiological role of the cannabinoid system. This revived the research on cannabinoid receptor antagonists which were expected to help answer these questions. The use of the cannabinoid agonist, THC, in its many preparations to enhance appetite is a well known fact. This fact led to the logical extension that blocking of the cannabinoid receptors might be useful in decreasing appetite and food intake. It was then discovered that the blockage of the CB1 receptor represented a new pharmacological target. The first specific CB1 receptor antagonist
/ inverse agonist
was rimonabant, discovered in 1994.
There are two main receptor types associated with the endocannabinoid signaling system; cannabinoid receptor 1
(CB1) and 2 (CB2
). Both receptors are 7-transmembrane G-protein coupled receptors (GPCRs) which inhibit the accumulation of cyclic adenosine monophosphate
within cells. CB1 receptors are present in highest concentration in the brain but can also be found in the periphery. CB2 receptors are mostly located in the immune and haematopoietic systems.
Endocannabinoids are eicosanoids acting as agonists for cannabinoid receptors and they occur naturally in the body. Cannabinoid receptor-related processes are for example involved in cognition, memory, anxiety, control of appetite, emesis, motor behavior, sensory
, autonomic
and neuroendocrine responses, immune responses and inflammatory effects.
There are two well-characterized endocannabinoids located in the brain and periphery
. The first identified was anandamide
(arachidonoyl ethanolamide) and the second was 2-AG (2-arachidonoyl glycerol). Additional endocannabinoids include virodhamine
(O-arachidonoyl ethanolamine), noladin ether (2-arachidonoyl glyceryl ether) and NADA (N-arachidonoyl dopamine).
 CB1 receptors are coupled through Gi/o proteins and inhibit adenylyl cyclase
CB1 receptors are coupled through Gi/o proteins and inhibit adenylyl cyclase
and activate mitogen-activated protein (MAP) kinase. In addition, CB1 receptors inhibit presynaptic N- and P/Q-type calcium channels and activate inwardly rectifying potassium channels. CB1 antagonists produce inverse cannabimimetic effects that are opposite in direction from those produced by agonists for these receptors.
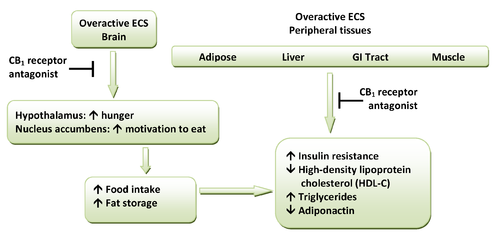
CB1 receptors are highly expressed in hypothalamic areas which are involved in central food intake control and feeding behavior. This strongly indicates that the cannabinoid system is directly involved in feeding regulation. These regions are also interconnected with the mesolimbic dopamine pathway, the so-called "reward" system. Therefore, CB1 antagonists might indirectly inhibit the dopamine-mediated rewarding properties of food. Peripheral CB1 receptors are located in the gastrointestinal (GI) tract, liver and in adipose tissue. In the GI, CB1 receptors are located on nerve terminals in the intestines. Endocannabinoids act at the CB1 receptors to increase hunger and promote feeding and it is speculated that they decrease intestinal peristalsis
and gastric emptying. Thus, antagonism at these receptors can inverse these effects. Also, in peripheral tissues, antagonism of CB1 receptors increases insulin
sensitivity and oxidation of fatty acids in muscles and the liver. A hypothetical scheme for the metabolic effects of CB1 receptor antagonists is shown in Figure 1.
which led to the discovery of aminoalkyl indole antagonists with some but limited success. As the search based on the structure of agonists was disappointing it was no surprise that the first potent and selective cannabinoid antagonist belonged to an entirely new chemical family. In 1994 the first selective cannabinoid antagonist, SR141716 (rimonabant), was introduced by Sanofi belonging to a family of 1,5-diarylpyrazoles.
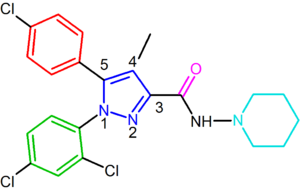
 Rimonabant, also known by the systematic name [N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1 H-pyrazole-3-carboxamidehydrochloride)], is a 1,5-diarylpyrazole CB1 receptor antagonist (Figure 2). Rimonabant is not only a potent and highly selective ligand of the CB1 receptor, but it is also orally active and antagonizes most of the effects of cannabinoid agonists, such as THC, both in vitro and in vivo. Rimonabant has shown clear clinical efficacy for the treatment of obesity.
Rimonabant, also known by the systematic name [N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1 H-pyrazole-3-carboxamidehydrochloride)], is a 1,5-diarylpyrazole CB1 receptor antagonist (Figure 2). Rimonabant is not only a potent and highly selective ligand of the CB1 receptor, but it is also orally active and antagonizes most of the effects of cannabinoid agonists, such as THC, both in vitro and in vivo. Rimonabant has shown clear clinical efficacy for the treatment of obesity.
will preferentially stabilize the inactive state (Figure 3).
Rimonabant has been reported in many cases to behave as an inverse agonist rather than as a neutral antagonist and it is likely that it binds preferentially to the inactive state of the CB1, thereby decreasing the activation of the signaling pathway. The key binding interaction is a hydrogen bond
formed between the carbonyl
group of rimonabant and the Lys192 residue of the CB1 receptor. This bond stabilizes the Lys192-Asp366 salt bridge
of the intracellular end of transmembrane helices 3 and 6 (Figure 4). This specific salt bridge is present in the inactive state of the receptor but absent in the active state.
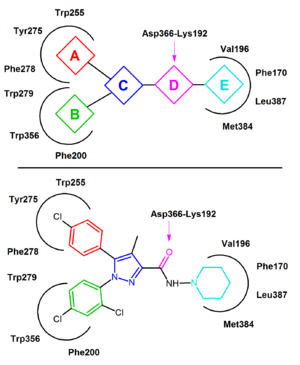
In the inactive state of CB1 rimonabant binds within the transmembrane-3-4-5-6 aromatic microdomain. The binding of rimonabant involves direct aromatic stacking interactions between its 2,4-dichlorophenyl ring and the Trp279/Phe200/Trp356 residues on the one side and the para-chlorophenyl ring and the Tyr275/Trp255/Phe278 residues on the other side. The lipophilic piperidinyl
moiety fits nicely in a cavity formed by the amino acid residues Val196/Phe170/Leu387 and Met384 (Figure 4).
s of rimonabant. A general CB1 inverse agonist pharmacophore
model can be extracted from the common features of these analogs, diarylpyrazoles (Figure 4). This pharmacophore contains a cyclic core, C, (e.g. pyrazole in rimonabant) substituted by two aromatic moieties, A and B. A hydrogen bond acceptor unit, D, connects C with a cyclic lipophilic part, E. In some cases unit E directly connects to C. In Figure 4 rimonabant is used as an example. Unit A represents a 4-chlorophenyl group and unit B a 2,4-dichlorophenyl ring. Unit C is the central pyrazole
ring and unit D represents the carbonyl group which serves as the hydrogen bond acceptor. Unit E represents a lipophilic aminopiperidinyl moiety.
A 2,4-dichloro-substituted phenyl ring at the pyrazole 1-position is preferred for affinity as well as for the activity. It has been shown that additional halogens on this phenyl ring decrease affinity.
It is also favorable to have a ring substitution at the 3-carboxamide group, such as the 1-piperidinyl group in rimonabant. Replacement of the amino piperidinyl substituent by alkyl amides, ethers, ketones, alcohols or alkanes resulted mostly in decreased affinity. Replacement of the piperidinyl by pentyl
or a heptyl chain gave the compounds agonistic properties. Based on these results it was concluded that the pyrazole 3-position seems to be involved in agonism, while the 1-,4-,5-positions appear to be involved in antagonism.
Research has shown that the absence of the carboxamide
oxygen results in decreased affinity. Furthermore, the presence of carboxamide oxygen contributes in conferring the inverse agonist properties, whereas analogs lacking this oxygen are found to be neutral antagonists. These results support the hypothesis that the carboxamide oxygen forms a hydrogen bond with Lys192 residue at the CB1 receptor.
(SAR) within this chemical group. While most compounds described are less potent than SR141716, two of them are worth mentioning, SR147778 and AM251
.
SR147778 (surinabant
), a second generation antagonist, has a longer duration of action than rimonabant and enhanced oral activity. This enhanced duration of action is probably due to the presence of the more metabolically stable ethyl group at the 4-position of its pyrazole ring. Another change is the replacement of the 5-phenyl chlorine substituent by bromine.
The diarylpyrazole derivative, AM251, has been described where chlorine substituent has been replaced by iodine in the para position of the 5-phenyl ring. This derivative appeared to be more potent and selective than rimonabant.
21 analogs possessing either an alkyl amide or an alkyl hydrazide
of variant lengths in position 3 were synthesized. It was observed that affinity increases with increased carbon chain length up to five carbons. Also the amide analogs exhibited higher affinity than hydrazide analogs. However, none of these analogs possessed significantly greater affinity than rimonabant but nevertheless, they were slightly more selective than rimonabant for the CB1 receptor over the CB2 receptor.
Several attempts have been made to increase the affinity of the diarylpyrazole derivatives by rigidifying the structure of rimonabant. In terms of the general pharmacophore model the units A, B and/or C are connected by additional bonds leading to rigid molecules. For example the condensed polycyclic pyrazole NESS-0327
showed 5000 times more affinity for the CB1 receptor than rimonabant. However, this compound possesses a poor central bioavailability
.
Another compound, the indazole
derivative O-1248, can be regarded as an analog of rimonabant wherein its 5-aryl group is fused to the pyrazole moiety. However, this structural modification resulted in a 67-fold decrease in CB1 receptor affinity.
These diarylpyrazole derivatives of rimonabant are summarized in Table 1.
), a potent CB1 antagonist which is about 1000-fold more selective for CB1 compared with CB2 and displays in vivo activity similar to rimonabant.
Another approach used to develop analogs of rimonabant was to replace central pyrazole ring by another heterocycle. An example of this approach are 4,5-diarylimidazoles and 1,5-diarylpyrrole-3-carboxamides.
A large number of fused bicyclic derivatives of diaryl-pyrazole and imidazoles have been reported. An example of these is a purine derivative where a pyrimidine
ring is fused to an imidazole
ring. Otenabant
(CP-945,598) is an example of a fused bicyclic derivative developed by Pfizer
.
Several research groups have studied six-membered ring pyrazole bioisostere
s. For example one 2,3-diarylpyridine derivative was shown to be potent and selective CB1 inverse agonist. The structure of this compound demonstrates the possibility that the amide moiety of rimonabant could be split into a lipophilic (benzyloxy) and a polar (nitrile) functionality. Other six-membered ring analogs are for example pyrimidines and pyrazines.
In addition to the five and six-membered ring analogs there are other cyclic derivatives such as the azetidines. One example is the methylsulfonamide azetidine derivative which has a 1,1-diaryl group that mimics the 1,5-diaryl moiety of the diarylpyrazoles. The sulfonyl
group serves as a hydrogen bond acceptor. The 1,1-diaryl group is also present in derivatives such as the benzodioxoles and hydantoins.
Acyclic analogs have also been reported. These analogs contain a 1,2-diaryl motif which corresponds to the 1,5-diaryl substituents of rimonabant. An example of an acyclic analog is taranabant
(MK-0364) developed by Merck
.
Representatives of these analogs are summarized in Table 2.
(EU) since June 2006 for the treatment of obesity. On 23rd of October 2008 the European Medicines Agency
(EMEA) has recommended the suspension of the marketing authorization across the EU for Acomplia from Sanofi-Aventis based on the risk of serious psychiatric disorders. On 5th of November 2008 Sanofi-Aventis announced discontinuation of rimonabant clinical development program.
Sanofi-Aventis has also discontinued development of surinabant (SR147778), a CB1 receptor antagonist for smoking cessation (31st of October 2008).
Merck has stated in its press release on 2nd of October 2008 that they will not seek regulatory approval for taranabant (MK-0364) to treat obesity and will discontinue its Phase III clinical development program. Data from Phase III clinical trial showed that greater efficacy and more adverse effects were associated with the higher doses of taranabant and it was determined that the overall profile of taranabant does not support further development for obesity.
Another pharmaceutical company, Pfizer, terminated the Phase III development program for its obesity compound otenabant (CP-945,598), a selective antagonist of the CB1 receptor. According to Pfizer their decision was based on changing regulatory perspectives on the risk/benefit profile of the CB1 class and likely new regulatory requirements for approval.
A number of initiatives have been published to develop CB1 antagonists that target only peripheral CB1 receptors by restricting their ability to cross the blood brain barrier. Among these initiatives 7TM Pharma has reported the development of TM38837
.
Receptor (biochemistry)
In biochemistry, a receptor is a molecule found on the surface of a cell, which receives specific chemical signals from neighbouring cells or the wider environment within an organism...
antagonists. The first cannabinoid receptor "antagonist," rimonabant
Rimonabant
Rimonabant is an anorectic antiobesity drug that has been withdrawn from the market. It is an inverse agonist for the cannabinoid receptor CB1...
, was described in 1994. Rimonabant blocks the CB1 receptor selectively and it has been shown to decrease food intake and regulate body-weight gain. The prevalence of obesity
Obesity
Obesity is a medical condition in which excess body fat has accumulated to the extent that it may have an adverse effect on health, leading to reduced life expectancy and/or increased health problems...
worldwide is increasing dramatically and has a great impact on public health. The lack of efficient and well-tolerated drugs to cure obesity has led to an increased interest in research and development of cannabinoid antagonists.
History

Hashish
Hashish is a cannabis preparation composed of compressed stalked resin glands, called trichomes, collected from the unfertilized buds of the cannabis plant. It contains the same active ingredients but in higher concentrations than unsifted buds or leaves...
and marijuana from the Indian hemp Cannabis sativa
Cannabis sativa
Cannabis sativa is an annual herbaceous plant in the Cannabaceae family. Humans have cultivated this herb throughout recorded history as a source of industrial fibre, seed oil, food, recreation, spiritual enlightenment and medicine...
L. have been used for medicinal and recreational purposes. In 1840, Schlesinger S. was apparently the first investigator to obtain an active extract from the leaves and flowers of hemp. A few years later, in 1848, Decourtive E. described the preparation of an ethanol extract that on evaporation of the solvent gave a dark resin, which he named “cannabin”.http://www.dialogues-cns.com/brochures/35/pdf/35.pdf#page=61. In 1964 the main active constituent of C. sativa L., Δ9-tetrahydrocannabinol (THC
THC
THC commonly refers to tetrahydrocannabinol, the main active chemical compound in Cannabis.THC may also refer to:* Tan Holdings Corporation...
), was isolated and synthesized by Mechoulam's
Raphael Mechoulam
Raphael Mechoulam is an Israeli professor of Medicinal Chemistry and Natural Products at the Hebrew University of Jerusalem in Israel...
laboratory. Two types of cannabinoid receptors, CB1 and CB2, responsible for the effects of THC were discovered and cloned in the early 1990s. Once cannabinoid receptors had been discovered, it became important to establish whether their agonists occur naturally in the body. This search led to the discovery of the first endogenous cannabinoid (endocannabinoid), anandamide
Anandamide
Anandamide, also known as N-arachidonoylethanolamide or AEA, is an endogenous cannabinoid neurotransmitter. The name is taken from the Sanskrit word ananda, which means "bliss, delight", and amide. It is synthesized from N-arachidonoyl phosphatidylethanolamine by multiple pathways...
(arachidonoyl ethanolamide). Later on other endocannabinoids were found, for example 2-AG (2-arachidonoyl glycerol). These findings raised further questions about the pharmacological and physiological role of the cannabinoid system. This revived the research on cannabinoid receptor antagonists which were expected to help answer these questions. The use of the cannabinoid agonist, THC, in its many preparations to enhance appetite is a well known fact. This fact led to the logical extension that blocking of the cannabinoid receptors might be useful in decreasing appetite and food intake. It was then discovered that the blockage of the CB1 receptor represented a new pharmacological target. The first specific CB1 receptor antagonist
Receptor antagonist
A receptor antagonist is a type of receptor ligand or drug that does not provoke a biological response itself upon binding to a receptor, but blocks or dampens agonist-mediated responses...
/ inverse agonist
Inverse agonist
In the field of pharmacology, an inverse agonist is an agent that binds to the same receptor as an agonist but induces a pharmacological response opposite to that agonist....
was rimonabant, discovered in 1994.
Endocannabinoids and their signaling system
The endogenous cannabinoid system includes cannabinoid receptors, their endogenous ligands (endocannabinoids) and enzymes for their synthesis and degradation.There are two main receptor types associated with the endocannabinoid signaling system; cannabinoid receptor 1
Cannabinoid receptor type 1
The cannabinoid receptor type 1, often abbreviated to CB1, is a G protein-coupled cannabinoid receptor located in the brain. It is activated by endocannabinoid neurotransmitters including anandamide and by the compound THC, found in the psychoactive drug cannabis.-Expression:The CB1 receptor is...
(CB1) and 2 (CB2
Cannabinoid receptor 2 (macrophage)
Cannabinoid receptor 2 , also known as CB2 or CNR2, is a G protein-coupled receptor from the cannabinoid receptor family, which in humans is encoded by the CNR2 gene...
). Both receptors are 7-transmembrane G-protein coupled receptors (GPCRs) which inhibit the accumulation of cyclic adenosine monophosphate
Cyclic adenosine monophosphate
Cyclic adenosine monophosphate is a second messenger important in many biological processes...
within cells. CB1 receptors are present in highest concentration in the brain but can also be found in the periphery. CB2 receptors are mostly located in the immune and haematopoietic systems.
Endocannabinoids are eicosanoids acting as agonists for cannabinoid receptors and they occur naturally in the body. Cannabinoid receptor-related processes are for example involved in cognition, memory, anxiety, control of appetite, emesis, motor behavior, sensory
Sensory system
A sensory system is a part of the nervous system responsible for processing sensory information. A sensory system consists of sensory receptors, neural pathways, and parts of the brain involved in sensory perception. Commonly recognized sensory systems are those for vision, hearing, somatic...
, autonomic
Autonomic nervous system
The autonomic nervous system is the part of the peripheral nervous system that acts as a control system functioning largely below the level of consciousness, and controls visceral functions. The ANS affects heart rate, digestion, respiration rate, salivation, perspiration, diameter of the pupils,...
and neuroendocrine responses, immune responses and inflammatory effects.
There are two well-characterized endocannabinoids located in the brain and periphery
Peripheral nervous system
The peripheral nervous system consists of the nerves and ganglia outside of the brain and spinal cord. The main function of the PNS is to connect the central nervous system to the limbs and organs. Unlike the CNS, the PNS is not protected by the bone of spine and skull, or by the blood–brain...
. The first identified was anandamide
Anandamide
Anandamide, also known as N-arachidonoylethanolamide or AEA, is an endogenous cannabinoid neurotransmitter. The name is taken from the Sanskrit word ananda, which means "bliss, delight", and amide. It is synthesized from N-arachidonoyl phosphatidylethanolamine by multiple pathways...
(arachidonoyl ethanolamide) and the second was 2-AG (2-arachidonoyl glycerol). Additional endocannabinoids include virodhamine
Virodhamine
Virodhamine is an endocannabinoid and a nonclassic eicosanoid, derived from arachidonic acid. O-Arachidonoyl ethanolamine is arachidonic acid and ethanolamine joined by an ester linkage, the opposite of the amide linkage found in anandamide...
(O-arachidonoyl ethanolamine), noladin ether (2-arachidonoyl glyceryl ether) and NADA (N-arachidonoyl dopamine).
Mechanism of action

Adenylate cyclase
Adenylate cyclase is part of the G protein signalling cascade, which transmits chemical signals from outside the cell across the membrane to the inside of the cell ....
and activate mitogen-activated protein (MAP) kinase. In addition, CB1 receptors inhibit presynaptic N- and P/Q-type calcium channels and activate inwardly rectifying potassium channels. CB1 antagonists produce inverse cannabimimetic effects that are opposite in direction from those produced by agonists for these receptors.
CB1 receptors are highly expressed in hypothalamic areas which are involved in central food intake control and feeding behavior. This strongly indicates that the cannabinoid system is directly involved in feeding regulation. These regions are also interconnected with the mesolimbic dopamine pathway, the so-called "reward" system. Therefore, CB1 antagonists might indirectly inhibit the dopamine-mediated rewarding properties of food. Peripheral CB1 receptors are located in the gastrointestinal (GI) tract, liver and in adipose tissue. In the GI, CB1 receptors are located on nerve terminals in the intestines. Endocannabinoids act at the CB1 receptors to increase hunger and promote feeding and it is speculated that they decrease intestinal peristalsis
Peristalsis
Peristalsis is a radially symmetrical contraction and relaxation of muscles which propagates in a wave down the muscular tube, in an anterograde fashion. In humans, peristalsis is found in the contraction of smooth muscles to propel contents through the digestive tract. Earthworms use a similar...
and gastric emptying. Thus, antagonism at these receptors can inverse these effects. Also, in peripheral tissues, antagonism of CB1 receptors increases insulin
Insulin
Insulin is a hormone central to regulating carbohydrate and fat metabolism in the body. Insulin causes cells in the liver, muscle, and fat tissue to take up glucose from the blood, storing it as glycogen in the liver and muscle....
sensitivity and oxidation of fatty acids in muscles and the liver. A hypothetical scheme for the metabolic effects of CB1 receptor antagonists is shown in Figure 1.
Drug design
The first approach to develop cannabinoid antagonists in the late 1980s was to modify the structure of THC but the results were disappointing. In the early 1990s new family of cannabinoid agonists was discovered from the NSAID (non-steroidal anti-inflammatory) drug pravadolinePravadoline
Pravadoline is an antiinflammatory and analgesic drug with an IC50 of 4.9 µM, related in structure to non-steroidal antinflammtory drugs such as indometacin...
which led to the discovery of aminoalkyl indole antagonists with some but limited success. As the search based on the structure of agonists was disappointing it was no surprise that the first potent and selective cannabinoid antagonist belonged to an entirely new chemical family. In 1994 the first selective cannabinoid antagonist, SR141716 (rimonabant), was introduced by Sanofi belonging to a family of 1,5-diarylpyrazoles.
Rimonabant



Binding
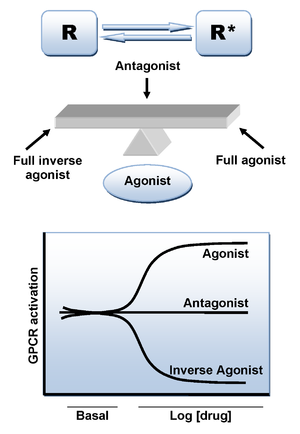
Binding of an agonist ligand to the CB1 receptor provokes a conformational change and leads to the active state of the receptor which is responsible for the signal transduction. However, there is an additional mechanism that can lead to the active state in the absence of ligand. As numerous other GPCRs, CB1 receptor displays a high level of constitutive activity and thus it can spontaneously adopt an active conformational state in the absence of agonist binding, keeping elevated basal levels of intracellular signaling. This can be explained by the two state-model of receptor activation in which receptors are in equilibrium between two states, active and inactive (R* and R). An agonist will stabilize the active state leading to activation, a neutral antagonist binds equally to active and inactive states, whereas an inverse agonistInverse agonist
In the field of pharmacology, an inverse agonist is an agent that binds to the same receptor as an agonist but induces a pharmacological response opposite to that agonist....
will preferentially stabilize the inactive state (Figure 3).
Rimonabant has been reported in many cases to behave as an inverse agonist rather than as a neutral antagonist and it is likely that it binds preferentially to the inactive state of the CB1, thereby decreasing the activation of the signaling pathway. The key binding interaction is a hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
formed between the carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group of rimonabant and the Lys192 residue of the CB1 receptor. This bond stabilizes the Lys192-Asp366 salt bridge
Salt bridge (protein)
Salt bridges fall into the broader category of noncovalent interactions. A salt bridge is actually a combination of two noncovalent interactions: hydrogen bonding and electrostatic interactions . This is most commonly observed to contribute stability to the entropically unfavorable folded...
of the intracellular end of transmembrane helices 3 and 6 (Figure 4). This specific salt bridge is present in the inactive state of the receptor but absent in the active state.
In the inactive state of CB1 rimonabant binds within the transmembrane-3-4-5-6 aromatic microdomain. The binding of rimonabant involves direct aromatic stacking interactions between its 2,4-dichlorophenyl ring and the Trp279/Phe200/Trp356 residues on the one side and the para-chlorophenyl ring and the Tyr275/Trp255/Phe278 residues on the other side. The lipophilic piperidinyl
Piperidine
Piperidine is an organic compound with the molecular formula 5NH. This heterocyclic amine consists of a six-membered ring containing five methylene units and one nitrogen atom...
moiety fits nicely in a cavity formed by the amino acid residues Val196/Phe170/Leu387 and Met384 (Figure 4).
Pharmacophore
Most CB1 antagonists reported so far are close analogs or isostereIsostere
Isosteres are molecules or ions with the same number of atoms and the same number of valence electrons.Some examples of isoteres include:*Sodium and hydrogen*Carbon dioxide and nitrous oxide *Silicon and carbon...
s of rimonabant. A general CB1 inverse agonist pharmacophore
Pharmacophore
thumb|right|300px|An example of a pharmacophore model.A pharmacophore is an abstract description of molecular features which are necessary for molecular recognition of a ligand by a biological macromolecule....
model can be extracted from the common features of these analogs, diarylpyrazoles (Figure 4). This pharmacophore contains a cyclic core, C, (e.g. pyrazole in rimonabant) substituted by two aromatic moieties, A and B. A hydrogen bond acceptor unit, D, connects C with a cyclic lipophilic part, E. In some cases unit E directly connects to C. In Figure 4 rimonabant is used as an example. Unit A represents a 4-chlorophenyl group and unit B a 2,4-dichlorophenyl ring. Unit C is the central pyrazole
Pyrazole
Pyrazole refers both to the class of simple aromatic ring organic compounds of the heterocyclic diazole series characterized by a 5-membered ring structure composed of three carbon atoms and two nitrogen atoms in adjacent positions, and to the unsubstituted parent compound...
ring and unit D represents the carbonyl group which serves as the hydrogen bond acceptor. Unit E represents a lipophilic aminopiperidinyl moiety.
Structure-activity relationships
Optimal binding at the CB1 receptor requires a para-substituted phenyl ring at the pyrazole 5-position. The 5-substituent of the pyrazole is involved in receptor recognition and antagonism. The para-substituent of the phenyl ring could be chlorine, bromine or iodine, but it has been shown that an alkyl chain could also be tolerated. Numbering of the central pyrazole ring is shown in Figure 2.A 2,4-dichloro-substituted phenyl ring at the pyrazole 1-position is preferred for affinity as well as for the activity. It has been shown that additional halogens on this phenyl ring decrease affinity.
It is also favorable to have a ring substitution at the 3-carboxamide group, such as the 1-piperidinyl group in rimonabant. Replacement of the amino piperidinyl substituent by alkyl amides, ethers, ketones, alcohols or alkanes resulted mostly in decreased affinity. Replacement of the piperidinyl by pentyl
Pentyl
In organic chemistry, pentyl is a five-carbon alkyl substituent with chemical formula -C5H11. It is the substituent form of the alkane pentane. In older literature, the common non-systematic name "amyl" was often used for the pentyl group....
or a heptyl chain gave the compounds agonistic properties. Based on these results it was concluded that the pyrazole 3-position seems to be involved in agonism, while the 1-,4-,5-positions appear to be involved in antagonism.
Research has shown that the absence of the carboxamide
Carboxamide
In organic chemistry carboxamides are functional groups with the general structure R-CO-NH2 with R as an organic substituent.Two amino acids, asparagine and glutamine, have a carboxamide group in them....
oxygen results in decreased affinity. Furthermore, the presence of carboxamide oxygen contributes in conferring the inverse agonist properties, whereas analogs lacking this oxygen are found to be neutral antagonists. These results support the hypothesis that the carboxamide oxygen forms a hydrogen bond with Lys192 residue at the CB1 receptor.
Diarylpyrazole derivatives
SR141716 (rimonabant) analogs have recently been described by several groups, leading to a good understanding of the structure-activity relationshipStructure-activity relationship
The structure–activity relationship is the relationship between the chemical or 3D structure of a molecule and its biological activity. The analysis of SAR enables the determination of the chemical groups responsible for evoking a target biological effect in the organism...
(SAR) within this chemical group. While most compounds described are less potent than SR141716, two of them are worth mentioning, SR147778 and AM251
AM251
AM-251 is an inverse agonist at the CB1 cannabinoid receptor. AM-251 is structurally very close to SR141716A , which both are biarylpyrazole cannabinoid receptor antagonists. In AM-251 the p-chloro group attached to the phenyl substituent at C-5 of the pyrazole ring is replaced with a p-iodo group...
.
SR147778 (surinabant
Surinabant
Surinabant is a cannabinoid receptor type 1 antagonist developed by Sanofi-Aventis. It is being investigated as a potential treatment for nicotine addiction, to assist smoking cessation...
), a second generation antagonist, has a longer duration of action than rimonabant and enhanced oral activity. This enhanced duration of action is probably due to the presence of the more metabolically stable ethyl group at the 4-position of its pyrazole ring. Another change is the replacement of the 5-phenyl chlorine substituent by bromine.
The diarylpyrazole derivative, AM251, has been described where chlorine substituent has been replaced by iodine in the para position of the 5-phenyl ring. This derivative appeared to be more potent and selective than rimonabant.
21 analogs possessing either an alkyl amide or an alkyl hydrazide
Hydrazide
Hydrazides in organic chemistry are a class of organic compounds sharing a common functional group characterized by a nitrogen to nitrogen covalent bond with 4 substituents with at least one of them being an acyl group. The general structure for an hydrazide is R2-N-N-R3R4. A related class of...
of variant lengths in position 3 were synthesized. It was observed that affinity increases with increased carbon chain length up to five carbons. Also the amide analogs exhibited higher affinity than hydrazide analogs. However, none of these analogs possessed significantly greater affinity than rimonabant but nevertheless, they were slightly more selective than rimonabant for the CB1 receptor over the CB2 receptor.
Several attempts have been made to increase the affinity of the diarylpyrazole derivatives by rigidifying the structure of rimonabant. In terms of the general pharmacophore model the units A, B and/or C are connected by additional bonds leading to rigid molecules. For example the condensed polycyclic pyrazole NESS-0327
NESS-0327
NESS-0327 is a drug used in scientific research which acts as an extremely potent and selective antagonist of the cannabinoid receptor CB1. It is much more potent an antagonist, and more selective for the CB1 receptor over CB2, than the more commonly used ligand rimonabant, with a Ki at CB1 of...
showed 5000 times more affinity for the CB1 receptor than rimonabant. However, this compound possesses a poor central bioavailability
Bioavailability
In pharmacology, bioavailability is a subcategory of absorption and is used to describe the fraction of an administered dose of unchanged drug that reaches the systemic circulation, one of the principal pharmacokinetic properties of drugs. By definition, when a medication is administered...
.
Another compound, the indazole
Indazole
Indazole, also called benzpyrazole or isoindazone, is a heterocyclic aromatic organic compound.Indazole derivatives display a broad variety of biological activities....
derivative O-1248, can be regarded as an analog of rimonabant wherein its 5-aryl group is fused to the pyrazole moiety. However, this structural modification resulted in a 67-fold decrease in CB1 receptor affinity.
These diarylpyrazole derivatives of rimonabant are summarized in Table 1.
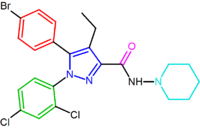 |
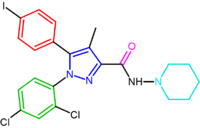 |
| SR147778 | AM251 AM251 AM-251 is an inverse agonist at the CB1 cannabinoid receptor. AM-251 is structurally very close to SR141716A , which both are biarylpyrazole cannabinoid receptor antagonists. In AM-251 the p-chloro group attached to the phenyl substituent at C-5 of the pyrazole ring is replaced with a p-iodo group... |
 |
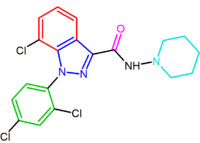 |
| NESS-0327 NESS-0327 NESS-0327 is a drug used in scientific research which acts as an extremely potent and selective antagonist of the cannabinoid receptor CB1. It is much more potent an antagonist, and more selective for the CB1 receptor over CB2, than the more commonly used ligand rimonabant, with a Ki at CB1 of... |
O-1248 |
Other derivatives
Structurally different from the 1,5-diarylpyrazoles are the chemical series of the 3,4-diarylpyrazolines. Within this series is SLV-319 (ibipinabantIbipinabant
Ibipinabant is a drug used in scientific research which acts as a potent and highly selective CB1 antagonist...
), a potent CB1 antagonist which is about 1000-fold more selective for CB1 compared with CB2 and displays in vivo activity similar to rimonabant.
Another approach used to develop analogs of rimonabant was to replace central pyrazole ring by another heterocycle. An example of this approach are 4,5-diarylimidazoles and 1,5-diarylpyrrole-3-carboxamides.
A large number of fused bicyclic derivatives of diaryl-pyrazole and imidazoles have been reported. An example of these is a purine derivative where a pyrimidine
Pyrimidine
Pyrimidine is a heterocyclic aromatic organic compound similar to benzene and pyridine, containing two nitrogen atoms at positions 1 and 3 of the six-member ring...
ring is fused to an imidazole
Imidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
ring. Otenabant
Otenabant
Otenabant is a drug which acts as a potent and highly selective CB1 antagonist. It was developed by Pfizer for the treatment of obesity, but development for this application has been discontinued following the problems seen during clinical use of the similar drug rimonabant....
(CP-945,598) is an example of a fused bicyclic derivative developed by Pfizer
Pfizer
Pfizer, Inc. is an American multinational pharmaceutical corporation. The company is based in New York City, New York with its research headquarters in Groton, Connecticut, United States...
.
Several research groups have studied six-membered ring pyrazole bioisostere
Bioisostere
In medicinal chemistry, bioisosteres are substituents or groups with similar physical or chemical properties which produce broadly similar biological properties to a chemical compound. In drug design, the purpose of exchanging one bioisostere for another is to enhance the desired biological or...
s. For example one 2,3-diarylpyridine derivative was shown to be potent and selective CB1 inverse agonist. The structure of this compound demonstrates the possibility that the amide moiety of rimonabant could be split into a lipophilic (benzyloxy) and a polar (nitrile) functionality. Other six-membered ring analogs are for example pyrimidines and pyrazines.
In addition to the five and six-membered ring analogs there are other cyclic derivatives such as the azetidines. One example is the methylsulfonamide azetidine derivative which has a 1,1-diaryl group that mimics the 1,5-diaryl moiety of the diarylpyrazoles. The sulfonyl
Sulfonyl
A sulfonyl group can refer either to a functional group found primarily in sulfones or to a substituent obtained from a sulfonic acid by the removal of the hydroxyl group similarly to acyl groups...
group serves as a hydrogen bond acceptor. The 1,1-diaryl group is also present in derivatives such as the benzodioxoles and hydantoins.
Acyclic analogs have also been reported. These analogs contain a 1,2-diaryl motif which corresponds to the 1,5-diaryl substituents of rimonabant. An example of an acyclic analog is taranabant
Taranabant
Taranabant is a cannabinoid receptor type 1 inverse agonist being investigated as a potential treatment for obesity due to its anorectic effects. It was discovered by Merck & Co....
(MK-0364) developed by Merck
Merck & Co.
Merck & Co., Inc. , also known as Merck Sharp & Dohme or MSD outside the United States and Canada, is one of the largest pharmaceutical companies in the world. The Merck headquarters is located in Whitehouse Station, New Jersey, an unincorporated area in Readington Township...
.
Representatives of these analogs are summarized in Table 2.
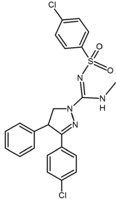 |
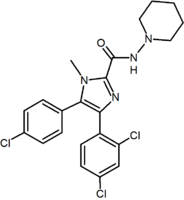 |
 |
|
| Type of derivative |
3,4-Diarylpyrazoline (SLV319) | 4,5-Diarylimidazole | 1,5-Diarylpyrrole-3-carboxamides |
 |
 |
 |
|
| Type of derivative |
Purine Purine A purine is a heterocyclic aromatic organic compound, consisting of a pyrimidine ring fused to an imidazole ring. Purines, including substituted purines and their tautomers, are the most widely distributed kind of nitrogen-containing heterocycle in nature.... (pyrimidine Pyrimidine Pyrimidine is a heterocyclic aromatic organic compound similar to benzene and pyridine, containing two nitrogen atoms at positions 1 and 3 of the six-member ring... ring fused to an imidazole Imidazole Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents... ring) |
Purine derivative (Otenabant Otenabant Otenabant is a drug which acts as a potent and highly selective CB1 antagonist. It was developed by Pfizer for the treatment of obesity, but development for this application has been discontinued following the problems seen during clinical use of the similar drug rimonabant.... ) |
2,3-Diarylpyridine |
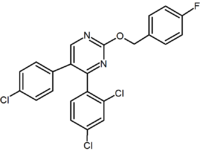 |
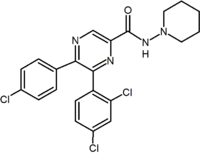 |
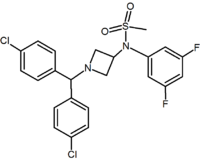 |
|
| Type of derivative |
Pyrimidine Pyrimidine Pyrimidine is a heterocyclic aromatic organic compound similar to benzene and pyridine, containing two nitrogen atoms at positions 1 and 3 of the six-member ring... |
Pyrazine Pyrazine Pyrazine is a heterocyclic aromatic organic compound with the chemical formula C4H4N2.Pyrazine is a symmetrical molecule with point group D2h. Derivatives like phenazine are well known for their antitumor, antibiotic and diuretic activity. Pyrazine is less basic in nature than pyridine, pyridazine... |
Methylsulfonamide azetidine Azetidine Azetidine is a heterocyclic organic compound. It belongs to the class of four membered rings and it contains a nitrogen atom.-External links:* *... |
 |
 |
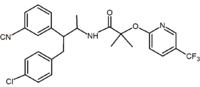 |
|
| Type of derivative |
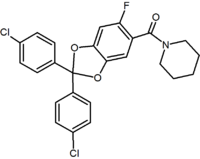
Benzodioxole | Hydantoin Hydantoin Hydantoin, which is also known as glycolylurea, is a heterocyclic organic compound that can be thought of as a cyclic "double-condensation reaction" product of glycolic acid and urea... |
Acyclic derivative (Taranabant Taranabant Taranabant is a cannabinoid receptor type 1 inverse agonist being investigated as a potential treatment for obesity due to its anorectic effects. It was discovered by Merck & Co.... ) |
Current status
Rimonabant (Acomplia) has been approved in the European UnionEuropean Union
The European Union is an economic and political union of 27 independent member states which are located primarily in Europe. The EU traces its origins from the European Coal and Steel Community and the European Economic Community , formed by six countries in 1958...
(EU) since June 2006 for the treatment of obesity. On 23rd of October 2008 the European Medicines Agency
European Medicines Agency
The European Medicines Agency is a European agency for the evaluation of medicinal products. From 1995 to 2004, the European Medicines Agency was known as European Agency for the Evaluation of Medicinal Products.Roughly parallel to the U.S...
(EMEA) has recommended the suspension of the marketing authorization across the EU for Acomplia from Sanofi-Aventis based on the risk of serious psychiatric disorders. On 5th of November 2008 Sanofi-Aventis announced discontinuation of rimonabant clinical development program.
Sanofi-Aventis has also discontinued development of surinabant (SR147778), a CB1 receptor antagonist for smoking cessation (31st of October 2008).
Merck has stated in its press release on 2nd of October 2008 that they will not seek regulatory approval for taranabant (MK-0364) to treat obesity and will discontinue its Phase III clinical development program. Data from Phase III clinical trial showed that greater efficacy and more adverse effects were associated with the higher doses of taranabant and it was determined that the overall profile of taranabant does not support further development for obesity.
Another pharmaceutical company, Pfizer, terminated the Phase III development program for its obesity compound otenabant (CP-945,598), a selective antagonist of the CB1 receptor. According to Pfizer their decision was based on changing regulatory perspectives on the risk/benefit profile of the CB1 class and likely new regulatory requirements for approval.
A number of initiatives have been published to develop CB1 antagonists that target only peripheral CB1 receptors by restricting their ability to cross the blood brain barrier. Among these initiatives 7TM Pharma has reported the development of TM38837
TM38837
TM38837 is a new small molecule inverse inhibitor of the CB1 receptor Cannabinoid receptor. It is being developed for the treatment of obesity and metabolic disorders by 7TM Pharma...
.
See also
- Cannabinoid receptor type 1Cannabinoid receptor type 1The cannabinoid receptor type 1, often abbreviated to CB1, is a G protein-coupled cannabinoid receptor located in the brain. It is activated by endocannabinoid neurotransmitters including anandamide and by the compound THC, found in the psychoactive drug cannabis.-Expression:The CB1 receptor is...
- Cannabinoid receptorCannabinoid receptorThe cannabinoid receptors are a class of cell membrane receptors under the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains...
- CannabinoidsCannabinoidsCannabinoids are a class of chemical compounds that include the phytocannabinoids , and chemical compounds that mimic the actions of phytocannabinoids or have a similar structure...
- Endocannabinoid systemEndocannabinoid systemThe endocannabinoid system refers to a group of neuromodulatory lipids and their receptors that are involved in a variety of physiological processes including appetite, pain-sensation, mood, and memory; it mediates the psychoactive effects of cannabis and, broadly speaking, includes:* The...
- RimonabantRimonabantRimonabant is an anorectic antiobesity drug that has been withdrawn from the market. It is an inverse agonist for the cannabinoid receptor CB1...

