
Addition polymerization
Encyclopedia
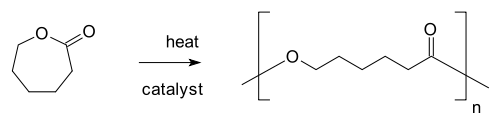
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
technique where unsaturated
Unsaturated compound
In organic chemistry, a saturated compound is a chemical compound that has of a chain of carbon atoms linked together by single bonds and has hydrogen atoms filling all of the other bonding orbitals of the carbon atoms. Alkanes are an example of saturated compounds...
monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
molecules add on to a growing polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
chain one at a time . It can be represented with the chemical equation
Chemical equation
A chemical equation is the symbolic representation of a chemical reaction where the reactant entities are given on the left hand side and the product entities on the right hand side. The coefficients next to the symbols and formulae of entities are the absolute values of the stoichiometric numbers...
:

where n is the degree of polymerization
Degree of polymerization
The degree of polymerization, or DP, is usually defined as the number of monomeric units in a macromolecule or polymer or oligomer molecule.For a homopolymer, there is only one type of monomeric unit andthe number-average degree of polymerization is given by...
.
"Chain growth polymerization" and addition polymerization (also called polyaddition) are two different concepts. In fact polyurethane
Polyurethane
A polyurethane is any polymer composed of a chain of organic units joined by carbamate links. Polyurethane polymers are formed through step-growth polymerization, by reacting a monomer with another monomer in the presence of a catalyst.Polyurethanes are...
polymerizes with addition polymerization (because its polymerization does not produce any small molecules, called "condensate"), but its reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
is a step-growth polymerization
Step-growth polymerization
Step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Many naturally occurring and some synthetic polymers are produced by step-growth...
.
The distinction between "addition polymerization" and "condensation polymerization" was introduced by Wallace Hume Carothers in 1929, and are referred to the type of products, respectively:
- a polymer only (addition)
- a polymer and a molecule with a low molecular weight (condensation)
The distinction between "step-growth polymerization" and "chain-growth polymerization" was instead introduced by Paul Flory
Paul Flory
Paul John Flory was an American chemist and Nobel laureate who was known for his prodigious volume of work in the field of polymers, or macromolecules...
in 1953, and are referred to the reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
s, respectively:
- by functional groups (step-growth polymerization)
- by free-radical or ion (chain-growth polymerization)
The main characteristics are:
- polymerization process takes place in three distinct steps:
- chain initiationInitiation (chemistry)In chemistry, initiation is a chemical reaction that triggers one or more secondary reactions. Often the initiation reaction generates a reactive intermediate from a stable molecule which is then involved in secondary reactions. In polymerisation, initiation is followed by a chain reaction and...
, usually by means of an initiatorRadical initiatorIn chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions . These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical initiators are utilized in industrial processes such...
which starts the chemical process. Typical initiators include any organic compound with a labile group: e.g. azo (-N=N-), disulfide (-S-S-), or peroxide (-O-O-). Two examples are benzoyl peroxideBenzoyl peroxideBenzoyl peroxide is an organic compound in the peroxide family. It consists of two benzoyl groups bridged by a peroxide link. Its structural formula is [C6H5C]2O2. It is one of the most important organic peroxides in terms of applications and the scale of its production...
and AIBN. - chain propagationChain propagationChain propagation is a process in which a reactive intermediate is continuously regenerated during the course of a chemical reaction. In polymerization reaction, the reactive end-groups of a polymer chain react in each propagation step with a new monomer molecule transferring the reactive group to...
- chain terminationChain terminationChain termination is any chemical reaction that ceases the formation of reactive intermediates in a chain propagation step in the course of a polymerization, effectively bringing it to a halt.- Mechanisms of Termination :...
, which occurs either by combination or disproportionationDisproportionationDisproportionation, also known as dismutation is used to describe a specific type of redox reaction in which a species is simultaneously reduced and oxidized so as to form two different products....
. Termination, in radical polymerization, is when the free radicals combine and is the end of the polymerization process.- some side reactions may occur, such as: chain transfer to monomer, chain transfer to solvent, and chain transfer to polymer.
- unlike condensation polymerization (also known as step-growth polymerizationStep-growth polymerizationStep-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Many naturally occurring and some synthetic polymers are produced by step-growth...
):- high molecular weight polymer is formed at low conversion
- no small molecules, such as H2OWaterWater is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, are eliminated in this process
- new monomer adds on the growing polymer chain via the reactive active centre which can be a
- free radical in radical polymerizationRadical polymerizationFree radical polymerization is a method of polymerization by which a polymer forms by the successive addition of free radical building blocks. Free radicals can be formed via a number of different mechanisms usually involving separate initiator molecules...
- carbocationCarbocationA carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
in cationic polymerization - carbanionCarbanionA carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
in anionic polymerization - organometallic complex in coordination polymerizationCoordination polymerizationCoordination polymerization is a form of addition polymerization in which monomer adds to a growing macromolecule through an organometallic active center...
.
- free radical in radical polymerization
- the monomer molecule can be a
- unsaturated compoundUnsaturated compoundIn organic chemistry, a saturated compound is a chemical compound that has of a chain of carbon atoms linked together by single bonds and has hydrogen atoms filling all of the other bonding orbitals of the carbon atoms. Alkanes are an example of saturated compounds...
like ethyleneEthyleneEthylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
or acetyleneAcetyleneAcetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
which make them reactive, see vinyl polymerVinyl polymerVinyl polymers are a group of polymers derived from vinyl monomers. Their backbone is an extended alkane chain, made by polymerizing an alkene group into a chain . In popular usage, "vinyl" refers only to polyvinyl chloride... - Alicyclic compoundAlicyclic compoundAn alicyclic compound is an organic compound that is both aliphatic and cyclic. They contain one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character...
, see ring-opening polymerizationRing-opening polymerizationIn polymer chemistry, ring-opening polymerization is a form of chain-growth polymerization, in which the terminal end of a polymer acts as a reactive center, where further cyclic monomers join to form a larger polymer chain through ionic propagation...
- unsaturated compound
- given special reactants and reaction conditions an addition polymerization can be considered a living polymerizationLiving polymerizationIn polymer chemistry, living polymerization is a form of addition polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is...
. - above a certain ceiling temperatureCeiling temperatureCeiling temperature is a measure of the tendency of polymers to revert to their monomers. When a polymer is at its ceiling temperature, the rate of polymerization and depolymerization of the polymer are equal. Generally, the ceiling temperature of a given polymer is correlated to the steric...
, no polymerization occurs.
Examples
- Benzoyl peroxideBenzoyl peroxideBenzoyl peroxide is an organic compound in the peroxide family. It consists of two benzoyl groups bridged by a peroxide link. Its structural formula is [C6H5C]2O2. It is one of the most important organic peroxides in terms of applications and the scale of its production...
is a radical initiatorRadical initiatorIn chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions . These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical initiators are utilized in industrial processes such...
for the free radical addition polymerization of styrene to produce polystyrenePolystyrenePolystyrene ) also known as Thermocole, abbreviated following ISO Standard PS, is an aromatic polymer made from the monomer styrene, a liquid hydrocarbon that is manufactured from petroleum by the chemical industry...
. - Aluminium chlorideAluminium chlorideAluminium chloride is the main compound of aluminium and chlorine. It is white, but samples are often contaminated with iron trichloride, giving it a yellow colour. The solid has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large...
is an initiator for the cationic addition polymerization of isobutyleneIsobutyleneIsobutylene is a hydrocarbon of significant industrial importance. It is a four-carbon branched alkene , one of the four isomers of butylene. At standard temperature and pressure it is a colorless flammable gas.-Uses:...
to form isobutyl synthetic rubberSynthetic rubberSynthetic rubber is is any type of artificial elastomer, invariably a polymer. An elastomer is a material with the mechanical property that it can undergo much more elastic deformation under stress than most materials and still return to its previous size without permanent deformation...
.

