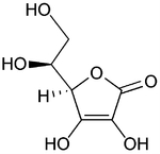
Sugar acids
Encyclopedia
Sugar acids are monosaccharide
s with a carboxyl group.
Main classes of sugar acids include:
Monosaccharide
Monosaccharides are the most basic units of biologically important carbohydrates. They are the simplest form of sugar and are usually colorless, water-soluble, crystalline solids. Some monosaccharides have a sweet taste. Examples of monosaccharides include glucose , fructose , galactose, xylose...
s with a carboxyl group.
Main classes of sugar acids include:
- Aldonic acidAldonic acidAn aldonic acid is any of a family of sugar acids obtained by oxidation of the aldehyde functional group of an aldose to form a carboxylic acid functional group. Thus, their general chemical formula is HOOC-n-CH2OH...
s, in which the aldehyde functional group of an aldoseAldoseAn aldose is a monosaccharide that contains only one aldehyde group per molecule. The chemical formula takes the form Cnn. The simplest possible aldose is the diose glycolaldehyde, which only contains two carbon atoms....
is oxidized - Ulosonic acidUlosonic acidAn ulosonic acid is a sugar acid obtained by the oxidation of the 1-hydroxyl group of a ketose to a carboxylic acid, creating an alpha-keto acid. An example of an ulosonic acid is ketodeoxyoctulosonic acid....
s, in which the first hydroxyl group of a 2-ketoseKetoseA ketose is a sugar containing one ketone group per molecule.With 3 carbon atoms, dihydroxyacetone is the simplest of all ketoses and is the only one having no optical activity. Ketoses can isomerize into an aldose when the carbonyl group is located at the end of the molecule...
is oxidised creating an α-ketoacid. - Uronic acidUronic acidthumb|300px|The [[Fischer projection]]s of [[glucose]] and [[glucuronic acid]]. Glucose's terminal carbon's hydroxyl group has been oxidized to a [[carboxylic acid]]....
s, in which the terminal hydroxyl group of an aldose or ketose is oxidized - Aldaric acidAldaric acidAldaric acids are a group of sugar acids, where the terminal hydroxyl groups of the sugars have been replaced by terminal carboxylic acids, and are characterised by the formula HOOC-n-COOH....
s, in which both ends of an aldose are oxidized
Examples
Examples of sugar acids include:- Aldonic acids
- Glyceric acidGlyceric acidGlyceric acid is a natural three-carbon sugar acid. Salts and esters of glyceric acid are known as glycerates.Biochemistry=Several phosphate derivatives of glyceric acid, including 2-phosphoglyceric acid, 3-phosphoglyceric acid, 2,3-bisphosphoglyceric acid, and 1,3-bisphosphoglyceric acid, are...
(3C) - Xylonic acidXylonic acidXylonic acid is a sugar acid that can be obtained by mild oxidation of xylose....
(5C) - Gluconic acidGluconic acidGluconic acid is an organic compound with molecular formula C6H12O7 and condensed structural formula HOCH24COOH. It is one of the 16 stereoisomers of 2,3,4,5,6-pentahydroxyhexanoic acid....
(6C) - Ascorbic acidAscorbic acidAscorbic acid is a naturally occurring organic compound with antioxidant properties. It is a white solid, but impure samples can appear yellowish. It dissolves well in water to give mildly acidic solutions. Ascorbic acid is one form of vitamin C. The name is derived from a- and scorbutus , the...
(6C, unsaturated lactone)
- Glyceric acid
- Ulosonic acids
- Neuraminic acidNeuraminic acidNeuraminic acid is a 9-carbon monosaccharide, a derivative of a ketononose. Neuraminic acid may be visualized as the product of an aldol-condensation product of pyruvic acid and D-mannosamine...
(5-amino-3,5-dideoxy-D-glyceroGlyceraldehydeGlyceraldehyde is a triose monosaccharide with chemical formula C3H6O3. It is the simplest of all common aldoses. It is a sweet, colorless, crystalline solid that is an intermediate compound in carbohydrate metabolism...
-D-galactoGalactoseGalactose , sometimes abbreviated Gal, is a type of sugar that is less sweet than glucose. It is a C-4 epimer of glucose....
-non-2-ulosonic acid) - Ketodeoxyoctulosonic acid (KDO or 3-deoxy-D-mannoMannoseMannose is a sugar monomer of the aldohexose series of carbohydrates. Mannose is a C-2 epimer of glucose. It is not part of human metabolism, but is a component of microbial cell walls, and is therefore a target of the immune system and also of antibiotics....
-oct-2-ulosonic acid)
- Neuraminic acid
- Uronic acids
- Glucuronic acidGlucuronic acidGlucuronic acid is a carboxylic acid. Its structure is similar to that of glucose. However, glucuronic acid's sixth carbon is oxidized to a carboxylic acid...
(6C) - Galacturonic acid (6C)
- Iduronic acidIduronic acidL-Iduronic acid is the major uronic acid component of the glycosaminoglycans dermatan sulfate, and heparin. It is also present in heparan sulfate although here in a minor amount relative to its carbon-5 epimer glucuronic acid....
(6C)
- Glucuronic acid
- Aldaric acids
- Tartaric acidTartaric acidTartaric acid is a white crystalline diprotic organic acid. It occurs naturally in many plants, particularly grapes, bananas, and tamarinds; is commonly combined with baking soda to function as a leavening agent in recipes, and is one of the main acids found in wine. It is added to other foods to...
(4C) - meso-Galactaric acid (Mucic acidMucic acidMucic acid, C6H10O8 or HOOC-4-COOH, is obtained by nitric acid oxidation of galactose or galactose-containing compounds like lactose, dulcite, quercite, and most varieties of gum....
) (6C) - D-Glucaric acid (Saccharic acidSaccharic acidSaccharic acid, also called glucaric acid, is a chemical compound with the formula C6H10O8. It is derived by oxidizing a sugar such as glucose with nitric acid.The salts of saccharic acid are called saccharates....
) (6C)
- Tartaric acid
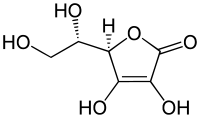 |

