
Mucic acid
Encyclopedia
Mucic acid, C6H10O8 or HOOC-(CHOH)4-COOH, (also known as galactaric or meso-galactaric acid) is obtained by nitric acid
oxidation of galactose
or galactose-containing compounds like lactose
, dulcite, quercite, and most varieties of gum
.
It forms a crystalline powder which melts at 213 °C. It is insoluble in alcohol
, and nearly insoluble in cold water. Due to the symmetry in the molecule, it is optically inactive
even though it has chiral
carbon atoms (i.e., it is a meso compound
). When heated with pyridine
to 140 °C, it is converted into allommic acid. When digested with fuming hydrochloric acid
for some time it is converted into a furfural
dicarboxylic acid
while on heating with barium
sulfide
it is transformed into athiophene
carboxylic acid. The ammonium salt yields on dry distillation carbon dioxide
, ammonia
, pyrrol and other substances. The acid when fused with caustic alkalis yields oxalic acid
.
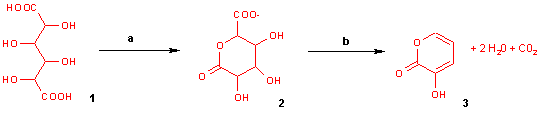
With potassium bisulfate mucic acid forms 3-hydroxy-2-pyrone by dehydration and decarboxylation.
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
oxidation of galactose
Galactose
Galactose , sometimes abbreviated Gal, is a type of sugar that is less sweet than glucose. It is a C-4 epimer of glucose....
or galactose-containing compounds like lactose
Lactose
Lactose is a disaccharide sugar that is found most notably in milk and is formed from galactose and glucose. Lactose makes up around 2~8% of milk , although the amount varies among species and individuals. It is extracted from sweet or sour whey. The name comes from or , the Latin word for milk,...
, dulcite, quercite, and most varieties of gum
Natural gum
Natural gums are polysaccharides of natural origin, capable of causing a large viscosity increase in solution, even at small concentrations. In the food industry they are used as thickening agents, gelling agents, emulsifying agents, and stabilizers...
.
It forms a crystalline powder which melts at 213 °C. It is insoluble in alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
, and nearly insoluble in cold water. Due to the symmetry in the molecule, it is optically inactive
Optical rotation
Optical rotation is the turning of the plane of linearly polarized light about the direction of motion as the light travels through certain materials. It occurs in solutions of chiral molecules such as sucrose , solids with rotated crystal planes such as quartz, and spin-polarized gases of atoms...
even though it has chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
carbon atoms (i.e., it is a meso compound
Meso compound
A meso compound or meso isomer is a non-optically active member of a set of stereoisomers, at least two of which are optically active. This means that despite containing two or more stereocenters it is not chiral. A meso compound is superimposable on its mirror image, and it does not produce a ""...
). When heated with pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
to 140 °C, it is converted into allommic acid. When digested with fuming hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
for some time it is converted into a furfural
Furfural
Furfural is an organic compound derived from a variety of agricultural byproducts, including corncobs, oat, wheat bran, and sawdust. The name furfural comes from the Latin word , meaning bran, referring to its usual source....
dicarboxylic acid
Dicarboxylic acid
Dicarboxylic acids are organic compounds that contain two carboxylic acid functional groups. In molecular formulae for dicarboxylic acids, these groups are often written as HOOC-R-COOH, where R may be an alkyl, alkenyl, alkynyl, or aryl group...
while on heating with barium
Barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. Barium is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with...
sulfide
Sulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
it is transformed into athiophene
Thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a flat five-membered ring, it is aromatic as indicated by its extensive substitution reactions. Related to thiophene are benzothiophene and dibenzothiophene, containing the thiophene ring fused with one and two benzene...
carboxylic acid. The ammonium salt yields on dry distillation carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
, pyrrol and other substances. The acid when fused with caustic alkalis yields oxalic acid
Oxalic acid
Oxalic acid is an organic compound with the formula H2C2O4. This colourless solid is a dicarboxylic acid. In terms of acid strength, it is about 3,000 times stronger than acetic acid. Oxalic acid is a reducing agent and its conjugate base, known as oxalate , is a chelating agent for metal cations...
.
With potassium bisulfate mucic acid forms 3-hydroxy-2-pyrone by dehydration and decarboxylation.


