
Aldose
Encyclopedia
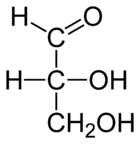
Monosaccharide
Monosaccharides are the most basic units of biologically important carbohydrates. They are the simplest form of sugar and are usually colorless, water-soluble, crystalline solids. Some monosaccharides have a sweet taste. Examples of monosaccharides include glucose , fructose , galactose, xylose...
(a simple sugar) that contains only one aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
(-CH=O) group per molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
. The chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
takes the form Cn(H2O)n. The simplest possible aldose is the diose
Diose
A diose is a monosaccharide containing two carbon atoms. Because the general chemical formula of an unmodified monosaccharide is n, where n is three or greater, it does not meet the formal definition of a monosaccharide. However, since it does fit the formula n, it is sometimes thought of as the...
glycolaldehyde
Glycolaldehyde
Glycolaldehyde is the smallest possible molecule that contains both an aldehyde group and a hydroxyl group. It is the only possible diose, a 2-carbon monosaccharide, although a diose is not strictly a saccharide...
, which only contains two carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s.
Because they have at least one asymmetric carbon centre, aldoses exhibit stereoisomerism
Stereoisomerism
Stereoisomers are isomeric molecules that have the same molecular formula and sequence of bonded atoms , but that differ only in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections...
. This means an aldose can exist in either a D form or L form of a Fischer projection
Fischer projection
The Fischer projection, devised by Hermann Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates and used by chemists, particularly in organic chemistry and...
. Biological systems tend to recognise D-aldoses more than L-aldoses.
An aldose differs from a ketose
Ketose
A ketose is a sugar containing one ketone group per molecule.With 3 carbon atoms, dihydroxyacetone is the simplest of all ketoses and is the only one having no optical activity. Ketoses can isomerize into an aldose when the carbonyl group is located at the end of the molecule...
in that it has a carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group at the end of the carbon chain instead of in the middle. This allows ketoses and aldoses to be chemically differentiated through Seliwanoff's test
Seliwanoff's test
Seliwanoff’s test is a chemical test which distinguishes between aldose and ketose sugars. Ketoses are distinguished from aldoses via their ketone/aldehyde functionality. If the sugar contains a ketone group, it is a ketose and if it contains an aldehyde group, it is an aldose...
. An aldose may isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
ize to a ketose through the Lobry-de Bruyn-van Ekenstein transformation
Lobry-de Bruyn-van Ekenstein transformation
In carbohydrate chemistry, the Lobry–de Bruyn–van Ekenstein transformation also known as the Lobry–de Bruyn–van-Alberda–van-Ekenstein transformation is the base or acid catalyzed transformation of an aldose into the ketose isomer or vice versa, with a tautomeric enediol as reaction intermediate....
.
List of aldoses
- DioseDioseA diose is a monosaccharide containing two carbon atoms. Because the general chemical formula of an unmodified monosaccharide is n, where n is three or greater, it does not meet the formal definition of a monosaccharide. However, since it does fit the formula n, it is sometimes thought of as the...
: glycolaldehydeGlycolaldehydeGlycolaldehyde is the smallest possible molecule that contains both an aldehyde group and a hydroxyl group. It is the only possible diose, a 2-carbon monosaccharide, although a diose is not strictly a saccharide... - TrioseTrioseA triose is a monosaccharide, or simple sugar, containing three carbon atoms. There are only three possible trioses: L-Glyceraldehyde and D-Glyceraldehyde, both aldotrioses because the carbonyl group is at the end of the chain, and dihydroxyacetone, a ketotriose because the carbonyl group is in...
: glyceraldehydeGlyceraldehydeGlyceraldehyde is a triose monosaccharide with chemical formula C3H6O3. It is the simplest of all common aldoses. It is a sweet, colorless, crystalline solid that is an intermediate compound in carbohydrate metabolism... - TetroseTetroseA tetrose is a monosaccharide with 4 carbon atoms. They have either an aldehyde functional group in position 1 or a ketone functional group in position 2 ....
s: erythroseErythroseErythrose is a tetrose carbohydrate with chemical formula C4H8O4. It has one aldehyde group and so is part of the aldose family. The natural isomer is D-erythrose....
, threoseThreoseThreose is a four-carbon monosaccharide or carbohydrate with molecular formula C4H8O4. It has a terminal aldehyde group rather than a ketone in its linear chain, and so is considered part of the aldose family of monosaccharides... - PentosePentoseA pentose is a monosaccharide with five carbon atoms. Pentoses are organized into two groups. Aldopentoses have an aldehyde functional group at position 1...
s: riboseRiboseRibose is an organic compound with the formula C5H10O5; specifically, a monosaccharide with linear form H––4–H, which has all the hydroxyl groups on the same side in the Fischer projection....
, arabinoseArabinoseArabinose is an aldopentose – a monosaccharide containing five carbon atoms, and including an aldehyde functional group.For biosynthetic reasons, most saccharides are almost always more abundant in nature as the "D"-form, or structurally analogous to D-glyceraldehyde.For sugars, the D/L...
, xyloseXyloseXylose is a sugar first isolated from wood, and named for it. Xylose is classified as a monosaccharide of the aldopentose type, which means that it contains five carbon atoms and includes an aldehyde functional group. It is the precursor to hemicellulose, one of the main constituents of biomass...
, lyxoseLyxoseLyxose is an aldopentose — a monosaccharide containing five carbon atoms, and including an aldehyde functional group. It has chemical formula 5105.Lyxose occurs only rarely in nature, for example, as a component of bacterial glycolipids.- See also :... - HexoseHexoseIn organic chemistry, a hexose is a monosaccharide with six carbon atoms, having the chemical formula C6H12O6. Hexoses are classified by functional group, with aldohexoses having an aldehyde at position 1, and ketohexoses having a ketone at position 2....
s: alloseAlloseAllose is an aldohexose sugar. It is a rare monosaccharide that has been isolated from the leaves of the African shrub Protea rubropilosa. It is soluble in water and practically insoluble in methanol.Allose is a C-3 epimer of glucose....
, altroseAltroseAltrose is an aldohexose sugar. D-Altrose is an unnatural monosaccharide. It is soluble in water and practically insoluble in methanol. However, L-altrose has been isolated from strains of the bacterium Butyrivibrio fibrisolvens....
, glucoseGlucoseGlucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
, mannoseMannoseMannose is a sugar monomer of the aldohexose series of carbohydrates. Mannose is a C-2 epimer of glucose. It is not part of human metabolism, but is a component of microbial cell walls, and is therefore a target of the immune system and also of antibiotics....
, guloseGuloseGulose is an aldohexose sugar. It is a monosaccharide that is very rare in nature, but has been found in archaea, bacteria and eukaryotes. It also exists as a syrup with a sweet taste. It is soluble in water and slightly soluble in methanol. Both the D- and L-forms are not fermentable by...
, idoseIdoseIdose is a hexose, a six carbon monosaccharide. It has an aldehyde group and is an aldose. It is not found in nature, but its uronic acid, iduronic acid, is important. It is a component of dermatan sulfate and heparan sulfate, which are glycosaminoglycans. The first and third hydroxyls point the...
, galactoseGalactoseGalactose , sometimes abbreviated Gal, is a type of sugar that is less sweet than glucose. It is a C-4 epimer of glucose....
, taloseTaloseTalose is an aldohexose sugar. It is an unnatural monosaccharide that is soluble in water and slightly soluble in methanol. Some etymologists suggest that talose's name derives from the automaton of Greek mythology named Talos, but the relevance is unclear....
, maltoseMaltoseMaltose , or malt sugar, is a disaccharide formed from two units of glucose joined with an αbond, formed from a condensation reaction. The isomer "isomaltose" has two glucose molecules linked through an α bond. Maltose is the second member of an important biochemical series of glucose chains....
, starchStarchStarch or amylum is a carbohydrate consisting of a large number of glucose units joined together by glycosidic bonds. This polysaccharide is produced by all green plants as an energy store...

