Pyridoxine 5’-phosphate oxidase
Encyclopedia
Pyridoxine 5’-phosphate oxidase is an enzyme
that catalyzes several reactions in the vitamin B6
metabolism pathway. Pyridoxine-5-P oxidase catalyzes the terminal step (also the rate-limiting step) in vitamin B6 metabolism, the biosynthesis of pyridoxal-5’-phosphate, the biologically active form of vitamin B6 which acts as an essential cofactor. Pyridoxine 5’-phosphate oxidase is a member of the enzyme class oxidase
s, or more specifically, oxidoreductases. These enzymes catalyze a simultaneous oxidation-reduction reaction. The substrate oxidase enzymes is hydroxlyated by one oxygen atom of molecular oxygen.
Concurrently, the other oxygen atom is reduced to water. Even though molecular oxygen is the electron acceptor in these enzymes’ reactions, they are unique because oxygen does not appear in the oxidized product.
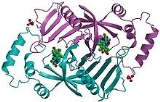
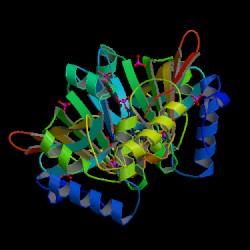
s. There are also salt-bridge interactions between the two monomers. Each subunit tightly binds one molecule of pyridoxal 5'-phosphate tightly on each subunit. Both alpha-helices and beta-sheets are present in the protein motif, which is best described as a split-barrel structure. This structure is due, in part, to the disulfide bonds present in the secondary protein structure of this enzyme. Multiple thiol groups (-SH) indicate the presence of disulfide bonds in the structure of the molecule. This enzyme requires the presence of a cofactor, FMN (flavin mononucleotide
).
Cofactors are ions or coenzymes necessary for enzyme activity. The FMN is located in a deep cleft (formed by the two polypeptide subunits), and held in place by extensive hydrogen-bond interactions with the protein. In this particular case, the FMN helps the enzyme to bind the substrates. In the absence of pyridoxal 5’-phosphate (PLP), the active site
of the enzyme is in an “open” conformation. Once substrate binds and is converted to PLP, the active site of the enzyme is in a partially “closed” conformation. Specific amino acid residues can form hydrogen bonds with the PLP, thus forming a lid that physically covers the active site, giving rise to the “closed” conformation.
, is a crucial nutrient for the human body, as it is responsible for more bodily functions that any other vitamin. Vitamin B6 is a coenzyme in the metabolism of carbohydrates, fats and proteins. This means that the enzymes which break these entities down for use in the body cannot function unless Vitamin B6 is present to induce a conformational change in the enzyme, thus activating it. Vitamin B6 also plays a role in the synthesis of hormones, red blood cells, neurotransmitters and enzymes. A person who is deficient in Vitamin B6 could suffer from insomnia, as well as suffer damage to the central nervous system.
of pyridoxamine-5'-phosphate and the deamination of pyridoxine 5-phosphate, both of which are key intermediates in the metabolism of B6. For a complete map of the B6 metabolism, http://www.genome.ad.jp/dbget-bin/show_pathway?map00750+1.4.3.5 Pyridoxine 5’-phosphate oxidase’s EC number is 1.4.3.5.
pyridoxamine 5'-phosphate + H2O + O2 = pyridoxal 5'-phosphate + NH3 + H2O2
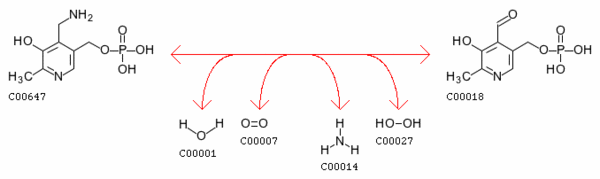
pyridoxine phosphate + oxygen <=> H2O2 + pyridoxal phosphate
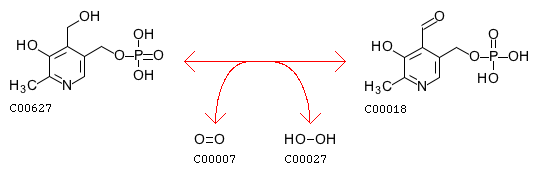
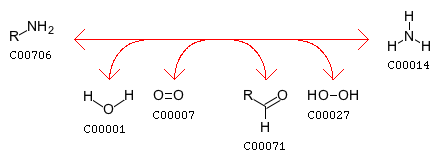
Pyridoxine 5’-phosphate oxidase also plays a role in nitrogen metabolism, converting amines to aldehydes NH3 by the reaction: amine + H2O + oxygen <=> aldehyde + NH3 + H2O2
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes several reactions in the vitamin B6
Vitamin B6
Vitamin B6 is a water-soluble vitamin and is part of the vitamin B complex group. Several forms of the vitamin are known, but pyridoxal phosphate is the active form and is a cofactor in many reactions of amino acid metabolism, including transamination, deamination, and decarboxylation...
metabolism pathway. Pyridoxine-5-P oxidase catalyzes the terminal step (also the rate-limiting step) in vitamin B6 metabolism, the biosynthesis of pyridoxal-5’-phosphate, the biologically active form of vitamin B6 which acts as an essential cofactor. Pyridoxine 5’-phosphate oxidase is a member of the enzyme class oxidase
Oxidase
An oxidase is any enzyme that catalyzes an oxidation-reduction reaction involving molecular oxygen as the electron acceptor. In these reactions, oxygen is reduced to water or hydrogen peroxide ....
s, or more specifically, oxidoreductases. These enzymes catalyze a simultaneous oxidation-reduction reaction. The substrate oxidase enzymes is hydroxlyated by one oxygen atom of molecular oxygen.
Concurrently, the other oxygen atom is reduced to water. Even though molecular oxygen is the electron acceptor in these enzymes’ reactions, they are unique because oxygen does not appear in the oxidized product.
Structure
Pyridoxine 5’-phosphate oxidase is a homodimer, or a molecule consisting of two identical polypeptide subunits. It is hypothesized that the two monomers are held together via disulfide bondDisulfide bond
In chemistry, a disulfide bond is a covalent bond, usually derived by the coupling of two thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry...
s. There are also salt-bridge interactions between the two monomers. Each subunit tightly binds one molecule of pyridoxal 5'-phosphate tightly on each subunit. Both alpha-helices and beta-sheets are present in the protein motif, which is best described as a split-barrel structure. This structure is due, in part, to the disulfide bonds present in the secondary protein structure of this enzyme. Multiple thiol groups (-SH) indicate the presence of disulfide bonds in the structure of the molecule. This enzyme requires the presence of a cofactor, FMN (flavin mononucleotide
Flavin mononucleotide
Flavin mononucleotide , or riboflavin-5′-phosphate, is a biomolecule produced from riboflavin by the enzyme riboflavin kinase and functions as prosthetic group of various oxidoreductases including NADH dehydrogenase as well as cofactor in biological blue-light photo receptors...
).
Cofactors are ions or coenzymes necessary for enzyme activity. The FMN is located in a deep cleft (formed by the two polypeptide subunits), and held in place by extensive hydrogen-bond interactions with the protein. In this particular case, the FMN helps the enzyme to bind the substrates. In the absence of pyridoxal 5’-phosphate (PLP), the active site
Active site
In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that...
of the enzyme is in an “open” conformation. Once substrate binds and is converted to PLP, the active site of the enzyme is in a partially “closed” conformation. Specific amino acid residues can form hydrogen bonds with the PLP, thus forming a lid that physically covers the active site, giving rise to the “closed” conformation.

Pathway
Pyridoxine 5’-phosphate oxidase is the enzyme that catalyzes the rate-limited step of the B6 metabolism pathway. Vitamin B6, which is also known as pyridoxinePyridoxine
Pyridoxine is one of the compounds that can be called vitamin B6, along with pyridoxal and pyridoxamine. It differs from pyridoxamine by the substituent at the '4' position. It is often used as 'pyridoxine hydrochloride'.-Chemistry:...
, is a crucial nutrient for the human body, as it is responsible for more bodily functions that any other vitamin. Vitamin B6 is a coenzyme in the metabolism of carbohydrates, fats and proteins. This means that the enzymes which break these entities down for use in the body cannot function unless Vitamin B6 is present to induce a conformational change in the enzyme, thus activating it. Vitamin B6 also plays a role in the synthesis of hormones, red blood cells, neurotransmitters and enzymes. A person who is deficient in Vitamin B6 could suffer from insomnia, as well as suffer damage to the central nervous system.
Reactions
Pyridoxine 5’-phosphate oxidase catalyzes several reactions; the two most important are the deaminationDeamination
Deamination is the removal of an amine group from a molecule. Enzymes which catalyse this reaction are called deaminases.In the human body, deamination takes place primarily in the liver, however glutamate is also deaminated in the kidneys. Deamination is the process by which amino acids are...
of pyridoxamine-5'-phosphate and the deamination of pyridoxine 5-phosphate, both of which are key intermediates in the metabolism of B6. For a complete map of the B6 metabolism, http://www.genome.ad.jp/dbget-bin/show_pathway?map00750+1.4.3.5 Pyridoxine 5’-phosphate oxidase’s EC number is 1.4.3.5.
pyridoxamine 5'-phosphate + H2O + O2 = pyridoxal 5'-phosphate + NH3 + H2O2

pyridoxine phosphate + oxygen <=> H2O2 + pyridoxal phosphate

Pyridoxine 5’-phosphate oxidase also plays a role in nitrogen metabolism, converting amines to aldehydes NH3 by the reaction: amine + H2O + oxygen <=> aldehyde + NH3 + H2O2


