
Proline racemase
Encyclopedia
In enzymology, a proline racemase is an enzyme
that catalyzes
the chemical reaction
Hence, this enzyme has two substrates, L- and D-proline
, and two product
s, D- and L- proline.
This enzyme belongs to the family of proline racemases acting on free amino acid
s. The systematic name of this enzyme class is proline racemase. This enzyme participates in arginine
and proline
metabolism. These enzymes catalyse the interconversion of L- and D-proline in bacteria.
and fully characterized . The parasite enzyme, TcPRAC, is as a co-factor-independent proline racemase and displays B-cell mitogenic properties when released by T. cruzi upon infection, contributing to parasite escape.
Novel proline racemases of medical and veterinary importance were described respectively in Clostridium difficile
and Trypanosoma vivax . These studies showed that a peptide motif used as a minimal pattern signature to identify putative proline racemases (motif III*) is insufficient stringent per se to discriminate proline racemases from 4-hydroxyproline epimerase
s (HyPRE). Also, additional, non-dissociated elements that account for the discrimination of these enzymes were identified, based for instance on polarity constraints imposed by specific residues of the catalytic pockets. Based on those elements, enzymes incorrectly described as proline racemases were biochemically proved to be hydroxyproline epimerases (i.e. HyPREs from Pseudomonas aeruginosa
(Q9I476), Burkholderia pseudomallei
, Brucella abortus , Brucella suis and Brucella melitensis
.
then showed that two molecules of PYC associate with TcPRAC in solution, and that this association is time-dependent and most probably based on mechanism of negative cooperativity. Complementary biochemical findings are consistent with the presence of two active catalytic sites per homodimer, each pertaining to one enzyme subunit, challenging the previously proposed mechanism of one catalytic site per homodimer previously proposed.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- L-proline
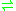 D-proline
D-proline
Hence, this enzyme has two substrates, L- and D-proline
Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
, and two product
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
s, D- and L- proline.
This enzyme belongs to the family of proline racemases acting on free amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s. The systematic name of this enzyme class is proline racemase. This enzyme participates in arginine
Arginine
Arginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during...
and proline
Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
metabolism. These enzymes catalyse the interconversion of L- and D-proline in bacteria.
Species distribution
This first eukaryotic proline racemase was identified in Trypanosoma cruziTrypanosoma cruzi
Trypanosoma cruzi is a species of parasitic euglenoid trypanosomes. This species causes the trypanosomiasis diseases in humans and animals in America...
and fully characterized . The parasite enzyme, TcPRAC, is as a co-factor-independent proline racemase and displays B-cell mitogenic properties when released by T. cruzi upon infection, contributing to parasite escape.
Novel proline racemases of medical and veterinary importance were described respectively in Clostridium difficile
Clostridium difficile
Clostridium difficile , also known as "CDF/cdf", or "C...
and Trypanosoma vivax . These studies showed that a peptide motif used as a minimal pattern signature to identify putative proline racemases (motif III*) is insufficient stringent per se to discriminate proline racemases from 4-hydroxyproline epimerase
4-hydroxyproline epimerase
In enzymology, a 4-hydroxyproline epimerase is an enzyme that catalyzes the chemical reactionHence, this enzyme has one substrate, trans-4-hydroxy-L-proline, and one product, cis-4-hydroxy-D-proline....
s (HyPRE). Also, additional, non-dissociated elements that account for the discrimination of these enzymes were identified, based for instance on polarity constraints imposed by specific residues of the catalytic pockets. Based on those elements, enzymes incorrectly described as proline racemases were biochemically proved to be hydroxyproline epimerases (i.e. HyPREs from Pseudomonas aeruginosa
Pseudomonas aeruginosa
Pseudomonas aeruginosa is a common bacterium that can cause disease in animals, including humans. It is found in soil, water, skin flora, and most man-made environments throughout the world. It thrives not only in normal atmospheres, but also in hypoxic atmospheres, and has, thus, colonized many...
(Q9I476), Burkholderia pseudomallei
Burkholderia pseudomallei
Burkholderia pseudomallei is a Gram-negative, bipolar, aerobic, motile rod-shaped bacterium. It infects humans and animals and causes the disease melioidosis. It is also capable of infecting plants....
, Brucella abortus , Brucella suis and Brucella melitensis
Brucella melitensis
-Introduction:Brucella melitensis is a gram negative coccobacillus bacteria from the Brucellaceae family. The bacterium causes Ovine Brucellosis, along with Brucella ovis. It can infect sheep, cattle, and sometimes humans and it can be transmitted by the stable fly...
.
Structural studies
The biochemical mechanism of proline racemase was first put forward in the late sixties by Cardinale and Abeles using the Clostridium sticklandii enzyme, CsPRAC. The catalytic mechanism of proline racemase was late revisited by Buschiazzo, Goytia and collaborators that, in 2006, resolved the structure of the parasite TcPRAC co-crystallyzed with its known competitive inhibitor - pyrrole carboxylic acid (PYC). Those studies showed that each active enzyme contains two catalytic pockets. Isothermal titration calorimetryIsothermal Titration Calorimetry
Isothermal titration calorimetry is a physical technique used to determine the thermodynamic parameters of interactions in solution. It is most often used to study the binding of small molecules to larger macromolecules .-Thermodynamic measurements:ITC is a quantitative technique that can...
then showed that two molecules of PYC associate with TcPRAC in solution, and that this association is time-dependent and most probably based on mechanism of negative cooperativity. Complementary biochemical findings are consistent with the presence of two active catalytic sites per homodimer, each pertaining to one enzyme subunit, challenging the previously proposed mechanism of one catalytic site per homodimer previously proposed.

