Premelting
Encyclopedia
Premelting describes the fact that, even below its melting point (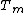 ), quasi-liquid films can be observed on crystalline surfaces. The thickness of the film is temperature (
), quasi-liquid films can be observed on crystalline surfaces. The thickness of the film is temperature (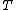 ) dependent. This effect is common for all crystalline materials.
) dependent. This effect is common for all crystalline materials.
Premelting shows its effects in e.g. frost heave, the growth of snowflakes
and, taking grain boundary interfaces into account, maybe even in the movement of glaciers.
Considering a solid-vapour interface, complete and incomplete premelting can be distinguished. During a temperature rise from below to above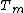 . In the case of complete premelting, the solid melts homogeneously from the outside to the inside. In the case of incomplete premelting the liquid film stays very thin during the beginning of the melting process, but droplets start to form on the interface. In either case, the solid always melts from the outside inwards, never from the inside.
. In the case of complete premelting, the solid melts homogeneously from the outside to the inside. In the case of incomplete premelting the liquid film stays very thin during the beginning of the melting process, but droplets start to form on the interface. In either case, the solid always melts from the outside inwards, never from the inside.
in 1842 for ice surfaces. He compared the effect which holds a snowball together to that which makes buildings from moistured sand stable. Another interesting thing he mentioned is that two blocks of ice can freeze together. Later Tammann and Stranski suggested that all surfaces might, due to the reduction of surface energy, start melting at their surfaces. Frenkel strengthened this by noting that, in contrast to liquids no overheating can be found for solids. After extensive studies on many materials it can be concluded that it is a common attribute of the solid state that the melting process begins at the surface.
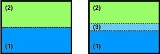
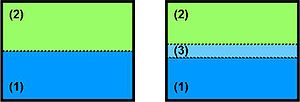
One always starts with looking at a crystalline solid phase (fig. 1: (1) solid) and another phase. This second phase (fig. 1: (2)) can either be vapour, liquid
or solid
. Further it can consist of the same chemical material or another. In the case of the second phase being a solid of the same chemical material one speaks of grain boundaries. This case is very important when looking at polycrystalline materials.
 In the following thermodynamical equilibrium is assumed, as well as for simplicity (2) should be a vaporous phase.
In the following thermodynamical equilibrium is assumed, as well as for simplicity (2) should be a vaporous phase.
The first (1) and the second (2) phase are always divided by some form of interface, what results in an interfacial energy
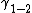 . One can now ask whether this energy can be lowered by inserting a third phase (l) in between (1) and (2). Written in interfacial energies this would mean:
. One can now ask whether this energy can be lowered by inserting a third phase (l) in between (1) and (2). Written in interfacial energies this would mean:
If this is the case then it is more efficient for the system to form a separating phase (3). The only possibility for the system to form such a layer is to take material of the solid and "melt" it to a quasi-liquid. In further notation there will be no distinction between quasi-liquid and liquid but one should always keep in mind that there is a difference. This difference to a real liquid becomes clear when looking at a very thin layer (l). As, due to the long range forces of the molecules of the solid material the liquid very near the solid still "feels" the order of crystalline solid and hence itself is in a state providing a not liquid like amount of order. As considering a very thin layer at the moment it is clear that the whole separating layer (l) is to well ordered for a liquid. Further comments on ordering can be found in the paragraph on Landau theory
.
Now, looking closer at the thermodynamics of the newly introduced phase (l), its Gibbs free energy
can be written as:
Were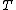 is the temperature,
is the temperature, 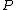 the pressure,
the pressure, 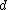 the thickness of (l) corresponding to the number or particles
the thickness of (l) corresponding to the number or particles 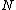 in this case.
in this case. 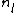 and
and 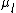 are the atomic density and the chemical potential in (l) and
are the atomic density and the chemical potential in (l) and 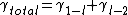 . Note that one has to consider that the interfacial energies can just be added to the Gibbs free energy in this case. As noted before
. Note that one has to consider that the interfacial energies can just be added to the Gibbs free energy in this case. As noted before  corresponds
corresponds  so the derivation to
so the derivation to  results in:
results in:
Where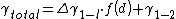 . Hence
. Hence 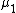 and
and 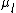 differ and
differ and 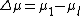 can be defined. Assuming that a Taylor expansion around the melting point
can be defined. Assuming that a Taylor expansion around the melting point 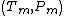 is possible and using the Clausius-Clapeyron equation one can get the following results:
is possible and using the Clausius-Clapeyron equation one can get the following results:
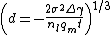
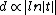
Where is in the order of molecular dimensions
is in the order of molecular dimensions 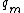 the specific melting heat and
the specific melting heat and 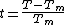
These formulas also show that the more the temperature increases, the more increases the thickness of the premelt as this is energetically advantageous. This is the explanation why no overheating
exists for this type of phase transition.
Theory on Casimir, respectively van der Waals, interactions of macroscopic bodies premelting can be viewed from an electrodynamical perspective.
A good example for determining the difference between complete and incomplete premelting is ice. From VUV frequencies on the polarizability of ice is greater than that of water, at lower frequencies this is vice versa. Assuming there is already a film of thickness d on the solid it is easy for any components for electromagnetic waves to travel through the film in the direction perpendicular to the solid surface as long d is small. Hence as long as the film is thin compared to the frequency interaction from the solid to the whole film is possible. But when d gets large against typical VUV frequencies the electronic structure of the film will be to slow to promote the high frequencies to the other end of the liquid phase. Thus this end of the liquid phase feels only a retarded van der Waals interaction from the solid phase. Hence the attraction between the liquid molecules themselves will outweigh and they will start forming droplets instead to thicken the film further. So the speed of light limits complete premelting.
This makes it a question of solid and surface free energies whether complete premelting occurs. Complete surface melting will occur when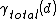 is monotonically decreasing. If
is monotonically decreasing. If 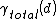 instead shows a global minimum at finite d than the premelting will be incomplete.
instead shows a global minimum at finite d than the premelting will be incomplete.
This implies: When the long range interactions in the system are attractive than there will be incomplete premelting - assuming the film thickness is larger than any repulsive interactions. Is the film thickness small compared to the range of the repulsive interactions present and the repulsive interactions are stronger than the attractive ones than complete premelting can occur.
For van der Waals interactions Lifshitz theory can now calculate which type of premelting should occur for a special system. In fact small differences in systems can affect the type of premelting. For example ice in an atmosphere of water vapour shows incomplete premelting, whereas the premelting of ice in air is complete.
For solid - solid interfaces it cannot be predicted in general whether the premelting is complete or incomplete when only considering van der Waals interactions. Here other types of interactions become very important. This also accounts for grain boundaries.
 Most insight in the problem probably emerges when approaching the effect form Landau Theory. Which is a little bit problematic as the melting of a bulk in general has to be considered as a first order phase transition, meaning the order parameter
Most insight in the problem probably emerges when approaching the effect form Landau Theory. Which is a little bit problematic as the melting of a bulk in general has to be considered as a first order phase transition, meaning the order parameter 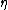 jumps at
jumps at 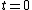 . The derivation of Lipowski (basic geometry shown in fig.2) leads to the following results when
. The derivation of Lipowski (basic geometry shown in fig.2) leads to the following results when 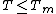 :
:
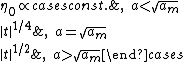
Where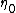 is the order parameter at the border between (2) and (l),
is the order parameter at the border between (2) and (l), 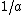 the so called extrapolation length and
the so called extrapolation length and 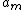 a constant that enters the model and has to be determined using experiment and other models. Hence one can see that the order parameter in the liquid film can undergo a continuous phase transition for large enough extrapolation length. A further result is that
a constant that enters the model and has to be determined using experiment and other models. Hence one can see that the order parameter in the liquid film can undergo a continuous phase transition for large enough extrapolation length. A further result is that 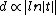 what corresponds to the result of the thermodynamical model in the case of short range interactions.
what corresponds to the result of the thermodynamical model in the case of short range interactions.
Landau Theory does not consider fluctuations like capillary waves, this could change the results qualitatively.
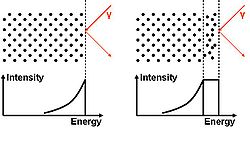 There are several techniques to prove the existence of a liquid layer on a well ordered surface. Basically it is all about showing that there is a phase on top of the solid which has hardly any order (quasi-liquid, see fig. order parameter). One possibility was done by Frenken and van der Veen using proton scattering on a lead (Pb) single crystal (110) surface. First the surface was atomically cleaned in [UHV], because one obviously has to have a very well ordered surface for such experiments. Than they did proton shadowing and blocking measurements. An ideal shadowing and blocking measurements results in an energy spectrum of the scattered protons that shows only a peak for the first surface layer and nothing else. Due to the non ideality of the experiment the spectrum also shows effects of the underlying layers. That means the spectrum is not one well defined peak but has a tail to lower energies due to protons scattered on deeper layers which results in losing energies because of stopping.
There are several techniques to prove the existence of a liquid layer on a well ordered surface. Basically it is all about showing that there is a phase on top of the solid which has hardly any order (quasi-liquid, see fig. order parameter). One possibility was done by Frenken and van der Veen using proton scattering on a lead (Pb) single crystal (110) surface. First the surface was atomically cleaned in [UHV], because one obviously has to have a very well ordered surface for such experiments. Than they did proton shadowing and blocking measurements. An ideal shadowing and blocking measurements results in an energy spectrum of the scattered protons that shows only a peak for the first surface layer and nothing else. Due to the non ideality of the experiment the spectrum also shows effects of the underlying layers. That means the spectrum is not one well defined peak but has a tail to lower energies due to protons scattered on deeper layers which results in losing energies because of stopping.
This is different for a liquid film on the surface: This film does hardly (to the meaning of hardly see Landau theory) have any order. So the effects of shadowing and blocking vanish what means all the liquid film contributes the same amount of scattered electrons to the signal. Therefore the peak does not only have a tail, but also becomes broadened.
During their measurements Frenken and van der Veen raised the temperature to the melting point and hence could show that with increasing temperature a disordered film formed on the surface in equilibrium with a still well ordered Pb crystal.
 . Fraction coefficients for ice skaters are around or below 0.005. So there is a need for a water film on the ice surface in order to explain the small friction appearing in ice skating. Three possible mechanisms could account for a film of liquid water on the ice surface:
. Fraction coefficients for ice skaters are around or below 0.005. So there is a need for a water film on the ice surface in order to explain the small friction appearing in ice skating. Three possible mechanisms could account for a film of liquid water on the ice surface:
To make it clear before: Until now scientific community is not sure which mechanism makes ice skating possible. And it could easily be that all three effects play a role but people want to know whether one effect could it all do on its own.
For several decades it was common to explain the low friction of the skates on ice by pressure melting. But till today several arguments were found which contradict this thesis. The strongest argument against pressure melting as the only effect responsible for the low friction on ice is that ice skating is still possible below temperatures of -20 °C under which ice can not be melted due to pressure anymore (see phase diagram). But pressure below about 0.2 GPa could still result in a lowering of the melting temperature.
Nowadays scientists mostly look for the origin of the water film in the two later effects and there are again arguments in favour or against the one or the other.
The thickness of the film is an argument which is all in favour of the friction argument, as these thicknesses could reach the orders of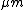 whereas film thickness due to premelting at lower temperatures, e.g. -10 °C, is in the orders of nm.
whereas film thickness due to premelting at lower temperatures, e.g. -10 °C, is in the orders of nm.
De Koning et al. found in their measurements that the adding of impurities to the ice can lower the friction coefficient in orders of 15%. When assuming that impurities do not play such a big role for friction melting, this indicates that a film of premelted water could play a certain role in ice skating. On the contrary they found that the friction coefficient increases with skating speed what would not support the friction argument, but could also be due to a different skating technique at higher speeds.
This is not the end of the story as there are several more arguments in favour or against premelting or friction. But one can summarize that the main problem of the premelting argument is the thin layer it provides. On the other hand one could say that 0.6 is a friction coefficient comparable to rubber and bitumen (roughly 0.8). Hence the question is whether one needs as much force to push a resting ice skater as pushing a resting normal person.
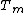 ), quasi-liquid films can be observed on crystalline surfaces. The thickness of the film is temperature (
), quasi-liquid films can be observed on crystalline surfaces. The thickness of the film is temperature (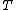 ) dependent. This effect is common for all crystalline materials.
) dependent. This effect is common for all crystalline materials.Premelting shows its effects in e.g. frost heave, the growth of snowflakes
Snowflakes
Snowflakes is the first Christmas album by American R&B singer–songwriter Toni Braxton, released in the United States on October 23, 2001 by Arista Records...
and, taking grain boundary interfaces into account, maybe even in the movement of glaciers.
Considering a solid-vapour interface, complete and incomplete premelting can be distinguished. During a temperature rise from below to above
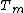 . In the case of complete premelting, the solid melts homogeneously from the outside to the inside. In the case of incomplete premelting the liquid film stays very thin during the beginning of the melting process, but droplets start to form on the interface. In either case, the solid always melts from the outside inwards, never from the inside.
. In the case of complete premelting, the solid melts homogeneously from the outside to the inside. In the case of incomplete premelting the liquid film stays very thin during the beginning of the melting process, but droplets start to form on the interface. In either case, the solid always melts from the outside inwards, never from the inside.History
The first to mention premelting might have been Michael FaradayMichael Faraday
Michael Faraday, FRS was an English chemist and physicist who contributed to the fields of electromagnetism and electrochemistry....
in 1842 for ice surfaces. He compared the effect which holds a snowball together to that which makes buildings from moistured sand stable. Another interesting thing he mentioned is that two blocks of ice can freeze together. Later Tammann and Stranski suggested that all surfaces might, due to the reduction of surface energy, start melting at their surfaces. Frenkel strengthened this by noting that, in contrast to liquids no overheating can be found for solids. After extensive studies on many materials it can be concluded that it is a common attribute of the solid state that the melting process begins at the surface.
Theoretical explanations
There are several ways to approach the topic of premelting the most figurative way might be thermodynamically. A more detailed or abstract view on what physics is important for premelting is given by the Lifshitz and the Landau theories.One always starts with looking at a crystalline solid phase (fig. 1: (1) solid) and another phase. This second phase (fig. 1: (2)) can either be vapour, liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
or solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
. Further it can consist of the same chemical material or another. In the case of the second phase being a solid of the same chemical material one speaks of grain boundaries. This case is very important when looking at polycrystalline materials.
Thermodynamical picture for solid gas interface

The first (1) and the second (2) phase are always divided by some form of interface, what results in an interfacial energy
Surface energy
Surface energy quantifies the disruption of intermolecular bonds that occur when a surface is created. In the physics of solids, surfaces must be intrinsically less energetically favorable than the bulk of a material, otherwise there would be a driving force for surfaces to be created, removing...
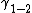 . One can now ask whether this energy can be lowered by inserting a third phase (l) in between (1) and (2). Written in interfacial energies this would mean:
. One can now ask whether this energy can be lowered by inserting a third phase (l) in between (1) and (2). Written in interfacial energies this would mean:
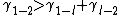 |
If this is the case then it is more efficient for the system to form a separating phase (3). The only possibility for the system to form such a layer is to take material of the solid and "melt" it to a quasi-liquid. In further notation there will be no distinction between quasi-liquid and liquid but one should always keep in mind that there is a difference. This difference to a real liquid becomes clear when looking at a very thin layer (l). As, due to the long range forces of the molecules of the solid material the liquid very near the solid still "feels" the order of crystalline solid and hence itself is in a state providing a not liquid like amount of order. As considering a very thin layer at the moment it is clear that the whole separating layer (l) is to well ordered for a liquid. Further comments on ordering can be found in the paragraph on Landau theory
Landau theory
Landau theory in physics was introduced by Lev Landau in an attempt to formulate a general theory of second-order phase transitions. He was motivated to suggest that the free energy of any system should obey two conditions: that the free energy is analytic, and that it obeys the symmetry of the...
.
Now, looking closer at the thermodynamics of the newly introduced phase (l), its Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
can be written as:
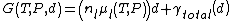 |
Were
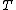 is the temperature,
is the temperature, 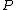 the pressure,
the pressure, 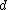 the thickness of (l) corresponding to the number or particles
the thickness of (l) corresponding to the number or particles 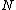 in this case.
in this case. 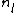 and
and 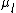 are the atomic density and the chemical potential in (l) and
are the atomic density and the chemical potential in (l) and 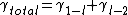 . Note that one has to consider that the interfacial energies can just be added to the Gibbs free energy in this case. As noted before
. Note that one has to consider that the interfacial energies can just be added to the Gibbs free energy in this case. As noted before 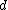 corresponds
corresponds 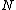 so the derivation to
so the derivation to 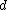 results in:
results in:
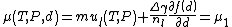 |
Where
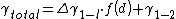 . Hence
. Hence 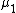 and
and 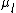 differ and
differ and 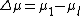 can be defined. Assuming that a Taylor expansion around the melting point
can be defined. Assuming that a Taylor expansion around the melting point 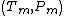 is possible and using the Clausius-Clapeyron equation one can get the following results:
is possible and using the Clausius-Clapeyron equation one can get the following results:
- For a long range potential assuming
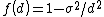 and
and 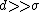 :
:
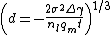
- For short range potential of the form
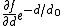 :
:
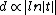
Where
 is in the order of molecular dimensions
is in the order of molecular dimensions 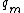 the specific melting heat and
the specific melting heat and 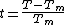
These formulas also show that the more the temperature increases, the more increases the thickness of the premelt as this is energetically advantageous. This is the explanation why no overheating
Overheating
Overheating may refer to:*Hyperthermia, also called sunstroke, an elevated body temperature due to failed thermoregulation*Thermal shock, the overheating of a device leading to reduced efficiency, damage or even destruction...
exists for this type of phase transition.
Lifshitz Theory: Complete and incomplete premelting
With the help of the LifshitzEvgeny Lifshitz
Evgeny Mikhailovich Lifshitz was a leading Soviet physicist of Jewish origin and the brother of physicist Ilya Mikhailovich Lifshitz. Lifshitz is well known in general relativity for coauthoring the BKL conjecture concerning the nature of a generic curvature...
Theory on Casimir, respectively van der Waals, interactions of macroscopic bodies premelting can be viewed from an electrodynamical perspective.
A good example for determining the difference between complete and incomplete premelting is ice. From VUV frequencies on the polarizability of ice is greater than that of water, at lower frequencies this is vice versa. Assuming there is already a film of thickness d on the solid it is easy for any components for electromagnetic waves to travel through the film in the direction perpendicular to the solid surface as long d is small. Hence as long as the film is thin compared to the frequency interaction from the solid to the whole film is possible. But when d gets large against typical VUV frequencies the electronic structure of the film will be to slow to promote the high frequencies to the other end of the liquid phase. Thus this end of the liquid phase feels only a retarded van der Waals interaction from the solid phase. Hence the attraction between the liquid molecules themselves will outweigh and they will start forming droplets instead to thicken the film further. So the speed of light limits complete premelting.
This makes it a question of solid and surface free energies whether complete premelting occurs. Complete surface melting will occur when
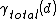 is monotonically decreasing. If
is monotonically decreasing. If 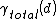 instead shows a global minimum at finite d than the premelting will be incomplete.
instead shows a global minimum at finite d than the premelting will be incomplete.This implies: When the long range interactions in the system are attractive than there will be incomplete premelting - assuming the film thickness is larger than any repulsive interactions. Is the film thickness small compared to the range of the repulsive interactions present and the repulsive interactions are stronger than the attractive ones than complete premelting can occur.
For van der Waals interactions Lifshitz theory can now calculate which type of premelting should occur for a special system. In fact small differences in systems can affect the type of premelting. For example ice in an atmosphere of water vapour shows incomplete premelting, whereas the premelting of ice in air is complete.
For solid - solid interfaces it cannot be predicted in general whether the premelting is complete or incomplete when only considering van der Waals interactions. Here other types of interactions become very important. This also accounts for grain boundaries.
Landau Theory

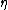 jumps at
jumps at 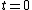 . The derivation of Lipowski (basic geometry shown in fig.2) leads to the following results when
. The derivation of Lipowski (basic geometry shown in fig.2) leads to the following results when 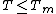 :
: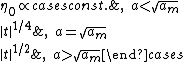
Where
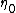 is the order parameter at the border between (2) and (l),
is the order parameter at the border between (2) and (l), 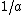 the so called extrapolation length and
the so called extrapolation length and 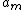 a constant that enters the model and has to be determined using experiment and other models. Hence one can see that the order parameter in the liquid film can undergo a continuous phase transition for large enough extrapolation length. A further result is that
a constant that enters the model and has to be determined using experiment and other models. Hence one can see that the order parameter in the liquid film can undergo a continuous phase transition for large enough extrapolation length. A further result is that 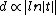 what corresponds to the result of the thermodynamical model in the case of short range interactions.
what corresponds to the result of the thermodynamical model in the case of short range interactions.Landau Theory does not consider fluctuations like capillary waves, this could change the results qualitatively.
Experimental proof for premelting

This is different for a liquid film on the surface: This film does hardly (to the meaning of hardly see Landau theory) have any order. So the effects of shadowing and blocking vanish what means all the liquid film contributes the same amount of scattered electrons to the signal. Therefore the peak does not only have a tail, but also becomes broadened.
During their measurements Frenken and van der Veen raised the temperature to the melting point and hence could show that with increasing temperature a disordered film formed on the surface in equilibrium with a still well ordered Pb crystal.
Curvature, disorder and impurities
Till now and ideal surface was considered but there are several effects which influence premelting:- Curvature: When the surface considered is not planar but exhibits a curvature premelting is effected. The rule is that whenever the surface is concave, viewed from the solid's perspective, than premelting is advanced. The fraction by which the thickness of the liquid film increases is given by:
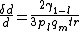 Where r is the local radius of the curved surface. Therefore it is also plausible that premelting starts in scratches or in corners of steps and hence has a flattening effect.
Where r is the local radius of the curved surface. Therefore it is also plausible that premelting starts in scratches or in corners of steps and hence has a flattening effect. - Disordered solids: As disorder in the solid increases its local free energy. The local chemical potential of the disordered solid lies above the chemical potential of the ordered solid. As in thermodynamic equilibrium the chemical potential of the premelted liquid film has to be equal to that of the disordered solid. It can be concluded that disorder in the solid phase causes the effect of premelting to increase.
- Impurities: Every winter again the melting point of ice is lowered with the help of salt. For premelting the situation is far more difficult than one would expect from that simple statement. It starts with the Lifshitz Theory which was roughly sketched above. But now the impurities cause screening in the liquid, they adsorb on the border between solid and liquid phase and all those effects make a general derivation of impurity effects impossible to state here. But it can be said that impurities have a great effect on the Temperature from which on premelting can be observed and they especially affect the thickness of the layer. What does not mean that the thickness is a monotone function in the concentration.
Ice skating
It should be noted first that the friction coefficient for ice, without a liquid film on the surface was measured to be . Fraction coefficients for ice skaters are around or below 0.005. So there is a need for a water film on the ice surface in order to explain the small friction appearing in ice skating. Three possible mechanisms could account for a film of liquid water on the ice surface:
. Fraction coefficients for ice skaters are around or below 0.005. So there is a need for a water film on the ice surface in order to explain the small friction appearing in ice skating. Three possible mechanisms could account for a film of liquid water on the ice surface:
- Pressure: Because of the abnormality of water ice can be melted by raising the pressure in the system
- Premelting: Due to premelting there is always a thin film of liquid water on the ice surface
- Friction: The sliding on the surface causes heat and melts the ice to a liquid water film
To make it clear before: Until now scientific community is not sure which mechanism makes ice skating possible. And it could easily be that all three effects play a role but people want to know whether one effect could it all do on its own.
For several decades it was common to explain the low friction of the skates on ice by pressure melting. But till today several arguments were found which contradict this thesis. The strongest argument against pressure melting as the only effect responsible for the low friction on ice is that ice skating is still possible below temperatures of -20 °C under which ice can not be melted due to pressure anymore (see phase diagram). But pressure below about 0.2 GPa could still result in a lowering of the melting temperature.
Nowadays scientists mostly look for the origin of the water film in the two later effects and there are again arguments in favour or against the one or the other.
The thickness of the film is an argument which is all in favour of the friction argument, as these thicknesses could reach the orders of
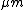 whereas film thickness due to premelting at lower temperatures, e.g. -10 °C, is in the orders of nm.
whereas film thickness due to premelting at lower temperatures, e.g. -10 °C, is in the orders of nm.De Koning et al. found in their measurements that the adding of impurities to the ice can lower the friction coefficient in orders of 15%. When assuming that impurities do not play such a big role for friction melting, this indicates that a film of premelted water could play a certain role in ice skating. On the contrary they found that the friction coefficient increases with skating speed what would not support the friction argument, but could also be due to a different skating technique at higher speeds.
This is not the end of the story as there are several more arguments in favour or against premelting or friction. But one can summarize that the main problem of the premelting argument is the thin layer it provides. On the other hand one could say that 0.6 is a friction coefficient comparable to rubber and bitumen (roughly 0.8). Hence the question is whether one needs as much force to push a resting ice skater as pushing a resting normal person.
External links
- http://phycomp.technion.ac.il/~phsorkin/thesis/node9.html Surface melting, Israel Institute of Technology
- http://www.lowtem.hokudai.ac.jp/ptdice/english/aletter.html Pattern of Snowflakes, Hokkaido University
- http://www.physicstoday.org/vol-58/iss-12/p50.shtml Robert Rosenberg: Why is Ice Slippery?; Physics Today, December 2005 (requires subscription)
- http://www.nytimes.com/2006/02/21/science/21ice.html Kenneth Chang: Explaining Ice: The Answers Are Slippery; The New York Times, February 21, 2006 (requires subscription)

