Phosphaalkene
Encyclopedia
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
s between carbon and phosphorus(III) with the formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
R2C=PR. In the compound phosphorine
Phosphorine
Phosphorine is a heavier element analog of pyridine, containing a phosphorus atom instead of an aza- moiety. It is also called phosphabenzene and belongs to the phosphaalkene class. Phosphorine is a planar aromatic compound with 88% of the aromaticity of that of benzene...
one carbon atom in benzene is replaced by phosphorus. The reactivity of phosphaalkenes is often compared to that of alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s and not to that of imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
s because the HOMO
Homo
Homo may refer to:*the Greek prefix ὅμο-, meaning "the same"*the Latin for man, human being*Homo, the taxonomical genus including modern humans...
of phosphaalkenes is not the phosphorus lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
(as in imines the amine lone pair) but the double bond. Therefore like alkenes, phosphaalkenes engage in Wittig reaction
Wittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
s, Peterson reactions, Cope rearrangement
Cope rearrangement
The Cope rearrangement is an extensively studied organic reaction involving the [3,3]-sigmatropic rearrangement of 1,5-dienes. It was developed by Arthur C. Cope...
s, and Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
s.
The first phosphaalkene discovered was a phosphabenzene, by Mërkl in 1969. The first localized phosphaalkene was reported in 1974 by Gerd Becker
Gerd Becker (chemist)
Gerd Becker is a German chemist.He holds a chair of inorganic chemistry at the University of Stuttgart. In 1974 he synthesized the first phosphaalkene.-External links:*...
as a keto-enol tautomerism
Keto-enol tautomerism
In organic chemistry, keto-enol tautomerism refers to a chemical equilibrium between a keto form and an enol . The enol and keto forms are said to be tautomers of each other...
akin a Brook rearrangement
Brook rearrangement
The Brook rearrangement in organic chemistry is a rearrangement reaction in which a organosilyl group switches position with a hydroxyl proton over a carbon to oxygen covalent bond under the influence of a base . It is named for the Canadian chemist Adrian Gibbs Brook...
:
In the same year Harold Kroto
Harold Kroto
Sir Harold Walter Kroto, FRS , born Harold Walter Krotoschiner, is a British chemist and one of the three recipients to share the 1996 Nobel Prize in Chemistry with Robert Curl and Richard Smalley....
established spectroscopically that thermolysis of Me2PH generates CH2=PMe. A general method for the synthesis of phosphaalkenes is by 1,2-elimination
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
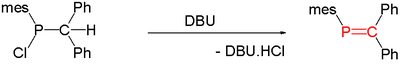
of suitable precursors, initiated thermally or by base such as DBU
DBU (chemistry)
1,8-Diazabicyclo[5.4.0]undec-7-ene, or more commonly DBU, is a chemical compound and belongs to the class of amidine compounds. It is used in organic synthesis as a catalyst and complexing ligand and a strong non-nucleophilic base.It is used as a curing agent for epoxy; it is used as a protecting...
, DABCO
DABCO
DABCO or 1,4-diazabicyclo[2.2.2]octane is a chemical compound. It is a polyurethane and Baylis-Hillman reaction catalyst, complexing ligand and Lewis base. It is used to regulate the reaction rate in Flexplay time-limited DVDs by adjusting pH. Antioxidants, like DABCO, are used to improve the...
or triethylamine
Triethylamine
Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation....
:
The Becker method is used in the synthesis of the phosphorus pendant of Poly(p-phenylene vinylene)
Poly(p-phenylene vinylene)
Poly is a conducting polymer of the rigid-rod polymer host family.PPV is the only polymer of this type that has so far been successfully processed into a highly ordered crystalline thin film. PPV and its derivatives are conducting polymers of rigid-rod polymer family...
:
-
- .
.svg.png)