Pfitzinger reaction
Encyclopedia
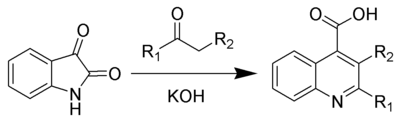
The Pfitzinger reaction (also known as the Pfitzinger-Borsche reaction) is the chemical reaction
of isatin
with base and a carbonyl
compound to yield substituted quinoline
-4-carboxylic acid
s.
 Several reviews have been published.
Several reviews have been published.
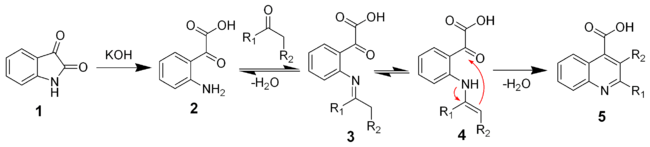 The reaction of isatin with a base such as potassium hydroxide
The reaction of isatin with a base such as potassium hydroxide
hydrolyses
the amide bond to give the keto-acid 2. This intermediate can be isolated, but is typically not. A ketone
(or aldehyde
) will react with the aniline to give the imine
(3) and the enamine
(4). The enamine will cyclize and dehydrate to give the desired quiniline (5).
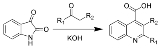
 Reaction of N-acyl
Reaction of N-acyl
isatins with base gives 2-hydroxy
-quinoline
-4-carboxylic acid
s.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
of isatin
Isatin
Isatin or 1H-indole-2,3-dione is an indole derivative. The compound was first obtained by Erdman and Laurent in 1841 as a product from the oxidation of indigo dye by nitric acid and chromic acids...
with base and a carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
compound to yield substituted quinoline
Quinoline
Quinoline is a heterocyclic aromatic organic compound. It has the formula C9H7N and is a colourless hygroscopic liquid with a strong odour. Aged samples, if exposed to light, become yellow and later brown...
-4-carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s.

Reaction mechanism

Potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
hydrolyses
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
the amide bond to give the keto-acid 2. This intermediate can be isolated, but is typically not. A ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
(or aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
) will react with the aniline to give the imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
(3) and the enamine
Enamine
An enamine is an unsaturated compound derived by the reaction of an aldehyde or ketone with a secondary amine followed by loss of H2O.The word "enamine" is derived from the affix en-, used as the suffix of alkene, and the root amine. This can be compared with enol, which is a functional group...
(4). The enamine will cyclize and dehydrate to give the desired quiniline (5).
Halberkann variant

Acyl
An acyl group is a functional group derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids.In organic chemistry, the acyl group is usually derived from a carboxylic acid . Therefore, it has the formula RCO-, where R represents an alkyl group that is...
isatins with base gives 2-hydroxy
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
-quinoline
Quinoline
Quinoline is a heterocyclic aromatic organic compound. It has the formula C9H7N and is a colourless hygroscopic liquid with a strong odour. Aged samples, if exposed to light, become yellow and later brown...
-4-carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s.
See also
- Camps quinoline synthesisCamps quinoline synthesisThe Camps quinoline synthesis is a chemical reaction whereby an o-acylaminoacetophenone is transformed into two different hydroxyquinolines using hydroxide ion....
- Friedländer synthesisFriedländer synthesisThe Friedländer synthesis is the chemical reaction of 2-aminobenzaldehydes with ketones to form quinoline derivatives. It is named after German chemist Paul Friedländer ....
- Niementowski quinazoline synthesisNiementowski quinazoline synthesisThe Niementowski quinazoline synthesis is the chemical reaction of anthranilic acids with amides to form 4-oxo-3,4-dihydroquinazolines.-References:# Stefan Niementowski, v. J. Prakt. Chem. 1895, 51, 564....

