
LeRoy radius
Encyclopedia
The LeRoy radius, derived by Robert J. LeRoy
, defines the internuclear distance between two atoms at which LeRoy-Bernstein
theory (sometimes called near-dissociation theory) becomes valid.
LeRoy-Bernstein theory is a semi-classical (WKB
) approach for describing vibrational energy levels
near the molecular dissociation limit. In this limit, the interaction potential between two atoms can be approximated as , which gives rise to a simple analytical approximation for the vibrational energy levels:
, which gives rise to a simple analytical approximation for the vibrational energy levels:
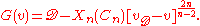
In this expression,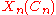 is a simple function depending only upon n and Cn, and
is a simple function depending only upon n and Cn, and  can be identified as an effective vibrational quantum number at dissociation.
can be identified as an effective vibrational quantum number at dissociation.
LeRoy later defined an expression for the radius that approximates a boundary between the region where electron exchange (quantum-mechanical
) terms are prominent, and the region where atoms and molecules approximately interact through the laws of classical physics
and, thus, LeRoy-Bernstein theory (as independent charge distributions and van der Waals interactions
expressible as a power series in the internuclear separation).
This radius is defined as
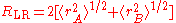 ,
,
where rA and rB denote the atomic radii
of the two atoms.
For , the internuclear potential can be reasonably approximated by charge independent atomic distributions, and the vibrational levels can be well described by LeRoy-Bernstein theory.
, the internuclear potential can be reasonably approximated by charge independent atomic distributions, and the vibrational levels can be well described by LeRoy-Bernstein theory.
For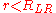 , there is no generally applicable expression for the internuclear potential. Likewise, there is no analogous expression for the vibrational level energies for this region and more sophisticated approximations
, there is no generally applicable expression for the internuclear potential. Likewise, there is no analogous expression for the vibrational level energies for this region and more sophisticated approximations
must be employed.
A derivation of a more general expression, called the m-dependent LeRoy radius, which depends on the magnetic quantum number
(m), was derived in 1995. This expression yields the traditional LeRoy Radius in the special case of a spherical, S-state, atom.
The LeRoy radius is described in high school level chemistry textbooks in Ontario (particularly, in Nelson Chemistry 12, which is the standard required textbook for grade 12 chemistry education in Ontario).
Robert J. LeRoy
Dr. Robert J. LeRoy is one of Canada’s leading chemists and is currently a University Professor at the University of Waterloo....
, defines the internuclear distance between two atoms at which LeRoy-Bernstein
Richard Barry Bernstein
Richard Barry Bernstein was an American physical chemist. He is primarily known for his researches in chemical kinetics and reaction dynamics by molecular beam scattering and laser techniques. He is credited with having founded femtochemistry, which laid the groundwork for developments in...
theory (sometimes called near-dissociation theory) becomes valid.
LeRoy-Bernstein theory is a semi-classical (WKB
WKB approximation
In mathematical physics, the WKB approximation or WKB method is a method for finding approximate solutions to linear partial differential equations with spatially varying coefficients...
) approach for describing vibrational energy levels
Molecular vibration
A molecular vibration occurs when atoms in a molecule are in periodic motion while the molecule as a whole has constant translational and rotational motion...
near the molecular dissociation limit. In this limit, the interaction potential between two atoms can be approximated as
 , which gives rise to a simple analytical approximation for the vibrational energy levels:
, which gives rise to a simple analytical approximation for the vibrational energy levels: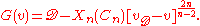
In this expression,
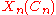 is a simple function depending only upon n and Cn, and
is a simple function depending only upon n and Cn, and  can be identified as an effective vibrational quantum number at dissociation.
can be identified as an effective vibrational quantum number at dissociation.LeRoy later defined an expression for the radius that approximates a boundary between the region where electron exchange (quantum-mechanical
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
) terms are prominent, and the region where atoms and molecules approximately interact through the laws of classical physics
Classical physics
What "classical physics" refers to depends on the context. When discussing special relativity, it refers to the Newtonian physics which preceded relativity, i.e. the branches of physics based on principles developed before the rise of relativity and quantum mechanics...
and, thus, LeRoy-Bernstein theory (as independent charge distributions and van der Waals interactions
Van der Waals force
In physical chemistry, the van der Waals force , named after Dutch scientist Johannes Diderik van der Waals, is the sum of the attractive or repulsive forces between molecules other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral...
expressible as a power series in the internuclear separation).
This radius is defined as
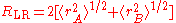 ,
,where rA and rB denote the atomic radii
Atomic radius
The atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the nucleus to the boundary of the surrounding cloud of electrons...
of the two atoms.
For
 , the internuclear potential can be reasonably approximated by charge independent atomic distributions, and the vibrational levels can be well described by LeRoy-Bernstein theory.
, the internuclear potential can be reasonably approximated by charge independent atomic distributions, and the vibrational levels can be well described by LeRoy-Bernstein theory.For
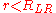 , there is no generally applicable expression for the internuclear potential. Likewise, there is no analogous expression for the vibrational level energies for this region and more sophisticated approximations
, there is no generally applicable expression for the internuclear potential. Likewise, there is no analogous expression for the vibrational level energies for this region and more sophisticated approximationsComputational chemistry
Computational chemistry is a branch of chemistry that uses principles of computer science to assist in solving chemical problems. It uses the results of theoretical chemistry, incorporated into efficient computer programs, to calculate the structures and properties of molecules and solids...
must be employed.
A derivation of a more general expression, called the m-dependent LeRoy radius, which depends on the magnetic quantum number
Magnetic quantum number
In atomic physics, the magnetic quantum number is the third of a set of quantum numbers which describe the unique quantum state of an electron and is designated by the letter m...
(m), was derived in 1995. This expression yields the traditional LeRoy Radius in the special case of a spherical, S-state, atom.
The LeRoy radius is described in high school level chemistry textbooks in Ontario (particularly, in Nelson Chemistry 12, which is the standard required textbook for grade 12 chemistry education in Ontario).

