
Krogmann's salt
Encyclopedia
Krogmann's salt is a mixed-valence
square planar
coordination complex of platinum
and cyanide
bonded through linear platinum metal chains, sometimes described as molecular wires.
Although the term Krogmann’s salt most commonly refers to a platinum metal complex of the formula K2[Pt(CN)4X0.3] where X is usually bromine
(or sometimes chlorine
), a number of non-stoichiometric metal salts containing the anionic complex [Pt(CN)4]2- can also be characterized under the blanket term “Krogmann’s salts.”
Modeled as an infinite one-dimensional molecular chain of platinum atoms, the high anisotropy
and restricted dimensionality of Krogmann’s salt and related compounds are becoming increasingly attractive properties for many facets of nanotechnology
.
in Germany
. Dr. Krogmann published the original journal article documenting the synthesis and characterization of the salt in 1969.
.
Krogmann’s salt has a tetragonal crystal structure with a Pt-Pt distance of 2.880 angstroms, which is much shorter than the metal-metal bond distances in other planar platinum complexes such as Ca[Pt(CN)4]·5H2O (3.36 angstroms), Sr[Pt(CN)4]·5H2O (3.58 angstroms), and Mg[Pt(CN)4]·7H2O (3.16 angstroms). The Pt-Pt distance in Krogmann's salt is only 0.1 angstroms longer than in platinum metal.
Each unit cell contains a site for Cl-, corresponding to 0.5 Cl- per Pt. However, this site is only filled 64% of the time, giving 0.32 Cl- per Pt in the actual compound. Because of this, the oxidation number
of Pt does not rise above +2.32.
Krogmann’s salt has no recognizable phase range and is characterized by broad and intense intervalence bands in its electronic spectra.
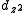 orbitals, Krogmann’s salt is an excellent conductor of electricity
orbitals, Krogmann’s salt is an excellent conductor of electricity
. This property makes it an attractive material for nanotechnology.
ratio mixture of the salts K2[Pt(CN)4] and K2[Pt(CN)4Br2] in water to give copper-colored needles of K2[Pt(CN)4]Br0.32·2.6 H2O.
Because excess PtII or PtIV complex crystallizes out with the product when the reactant ratio is changed, the product is therefore well defined, although non-stoichiometric.
Due to the explosion of nanotechnology in the last few years, many researchers have taken a renewed interest in Krogmann’s salt and its related compounds due to their high anisotropy, restricted dimensionality, and unique conductance properties.
A new group of platinum chains based on alternating cations and anions of [Pt(CNR)4]2+ (R = iPr, c-C12H23, p-(C2H5)C6H4) and [Pt(CN)4]2- is undergoing current research. These may be able to be used as vapochromic sensor
materials, or materials which change color when exposed to different vapors.
Similar to Krogmann’s platinum salt, it has been shown that it is possible to stabilize metal chains with only unsaturated
hydrocarbons, or olefins. Current research indicates that mononuclear Pd
0 and PdII react with conjugated
polyenes to give linear chains of Pd-Pd bonds protected by a “π-electron sheath.”
Not only do these olefin-stabilized metal chains constitute a significant contribution to the field of organometallic chemistry
, both the complex’s metal atom structures and the olefin ligands themselves can conduct a current. The prospect of creating molecular wires of conducting organic
and inorganic constituents has intriguing possibilities for future research, especially in microbiology
, nanotechnology, and organic circuitry.
Valence (chemistry)
In chemistry, valence, also known as valency or valence number, is a measure of the number of bonds formed by an atom of a given element. "Valence" can be defined as the number of valence bonds...
square planar
Square planar
The square planar molecular geometry in chemistry describes the stereochemistry that is adopted by certain chemical compounds...
coordination complex of platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
and cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
bonded through linear platinum metal chains, sometimes described as molecular wires.
Although the term Krogmann’s salt most commonly refers to a platinum metal complex of the formula K2[Pt(CN)4X0.3] where X is usually bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
(or sometimes chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
), a number of non-stoichiometric metal salts containing the anionic complex [Pt(CN)4]2- can also be characterized under the blanket term “Krogmann’s salts.”
Modeled as an infinite one-dimensional molecular chain of platinum atoms, the high anisotropy
Anisotropy
Anisotropy is the property of being directionally dependent, as opposed to isotropy, which implies identical properties in all directions. It can be defined as a difference, when measured along different axes, in a material's physical or mechanical properties An example of anisotropy is the light...
and restricted dimensionality of Krogmann’s salt and related compounds are becoming increasingly attractive properties for many facets of nanotechnology
Nanotechnology
Nanotechnology is the study of manipulating matter on an atomic and molecular scale. Generally, nanotechnology deals with developing materials, devices, or other structures possessing at least one dimension sized from 1 to 100 nanometres...
.
History
Krogmann’s salt was first synthesized by Dr. Klaus Krogmann in the late 1960s at the University of StuttgartUniversity of Stuttgart
The University of Stuttgart is a university located in Stuttgart, Germany. It was founded in 1829 and is organized in 10 faculties....
in Germany
Germany
Germany , officially the Federal Republic of Germany , is a federal parliamentary republic in Europe. The country consists of 16 states while the capital and largest city is Berlin. Germany covers an area of 357,021 km2 and has a largely temperate seasonal climate...
. Dr. Krogmann published the original journal article documenting the synthesis and characterization of the salt in 1969.
Structure and physical properties
Krogmann’s salt is a series of partially oxidized tetracyanoplatinate complexes linked by the platinum-platinum bonds on the top and bottom faces of the planar [Pt(CN)4]n- anions. This salt forms infinite stacks in the solid state based on the overlap of the dz2 orbitalsAtomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
.
Krogmann’s salt has a tetragonal crystal structure with a Pt-Pt distance of 2.880 angstroms, which is much shorter than the metal-metal bond distances in other planar platinum complexes such as Ca[Pt(CN)4]·5H2O (3.36 angstroms), Sr[Pt(CN)4]·5H2O (3.58 angstroms), and Mg[Pt(CN)4]·7H2O (3.16 angstroms). The Pt-Pt distance in Krogmann's salt is only 0.1 angstroms longer than in platinum metal.
Each unit cell contains a site for Cl-, corresponding to 0.5 Cl- per Pt. However, this site is only filled 64% of the time, giving 0.32 Cl- per Pt in the actual compound. Because of this, the oxidation number
Oxidation number
In coordination chemistry, the oxidation number of a central atom in a coordination compound is the charge that it would have if all the ligands were removed along with the electron pairs that were shared with the central atom. Oxidation numbers are often confused with oxidation states.The...
of Pt does not rise above +2.32.
Krogmann’s salt has no recognizable phase range and is characterized by broad and intense intervalence bands in its electronic spectra.
Chemical properties
One of the most widely researched properties of Krogmann’s salt is its unusual electric conductance. Because of its linear chain structure and overlap of the platinum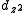 orbitals, Krogmann’s salt is an excellent conductor of electricity
orbitals, Krogmann’s salt is an excellent conductor of electricityElectricity
Electricity is a general term encompassing a variety of phenomena resulting from the presence and flow of electric charge. These include many easily recognizable phenomena, such as lightning, static electricity, and the flow of electrical current in an electrical wire...
. This property makes it an attractive material for nanotechnology.
Preparation
The usual preparation of Krogmann's salt involves the evaporation of a 5:1 molarMole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
ratio mixture of the salts K2[Pt(CN)4] and K2[Pt(CN)4Br2] in water to give copper-colored needles of K2[Pt(CN)4]Br0.32·2.6 H2O.
-
- 5K2[Pt(CN)4] + K2[Pt(CN)4Br2] + 15.6 H2O → 6K2[Pt(CN)4]Br0.32·2.6 H2O
Because excess PtII or PtIV complex crystallizes out with the product when the reactant ratio is changed, the product is therefore well defined, although non-stoichiometric.
Uses
Although there was a large body of research and literature generated on molecular wire-type metal complexes through the mid-1980s, interest in stacked metal-metal bonds saw a decline until only very recently.Due to the explosion of nanotechnology in the last few years, many researchers have taken a renewed interest in Krogmann’s salt and its related compounds due to their high anisotropy, restricted dimensionality, and unique conductance properties.
A new group of platinum chains based on alternating cations and anions of [Pt(CNR)4]2+ (R = iPr, c-C12H23, p-(C2H5)C6H4) and [Pt(CN)4]2- is undergoing current research. These may be able to be used as vapochromic sensor
Sensor
A sensor is a device that measures a physical quantity and converts it into a signal which can be read by an observer or by an instrument. For example, a mercury-in-glass thermometer converts the measured temperature into expansion and contraction of a liquid which can be read on a calibrated...
materials, or materials which change color when exposed to different vapors.
Similar to Krogmann’s platinum salt, it has been shown that it is possible to stabilize metal chains with only unsaturated
Saturation (chemistry)
In chemistry, saturation has six different meanings, all based on reaching a maximum capacity...
hydrocarbons, or olefins. Current research indicates that mononuclear Pd
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
0 and PdII react with conjugated
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
polyenes to give linear chains of Pd-Pd bonds protected by a “π-electron sheath.”
Not only do these olefin-stabilized metal chains constitute a significant contribution to the field of organometallic chemistry
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
, both the complex’s metal atom structures and the olefin ligands themselves can conduct a current. The prospect of creating molecular wires of conducting organic
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
and inorganic constituents has intriguing possibilities for future research, especially in microbiology
Microbiology
Microbiology is the study of microorganisms, which are defined as any microscopic organism that comprises either a single cell , cell clusters or no cell at all . This includes eukaryotes, such as fungi and protists, and prokaryotes...
, nanotechnology, and organic circuitry.

