
Geiger-Nuttall law
Encyclopedia
In nuclear physics
, the Geiger–Nuttall law or Geiger–Nuttall rule relates the decay constant of a radioactive isotope
with the energy of the alpha particles emitted. Roughly speaking, it states that short-lived isotopes emit more energetic alpha particles than long-lived ones.
The relationship also shows that half-lives are exponentially dependent on decay energy, so that very large changes in half-life make comparatively small differences in decay energy, and thus alpha particle energy. In practice, this means that alpha particles from all alpha-emitting isotopes across many orders of magnitude of difference in half-life, all nevertheless have about the same decay energy.
Formulated in 1911 by Hans Geiger and John Mitchell Nuttall
, in its modern form the Geiger–Nuttall law is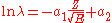
where λ is the decay constant (λ = ln2/half-life), Z the atomic number
, E the total kinetic energy
(of the alpha particle and the daughter nucleus), and a1 and a2 are constant
s.
in the atomic nucleus as a particle in a box
. The particle is in a bound state
because of the presence of the strong interaction
potential. It will constantly bounce from one side to the other, and due to the possibility of quantum tunneling by the wave though the potential barrier, each time it bounces, there will be a small likelihood for it to escape.
A knowledge of this quantum mechanical effect enables one to obtain this law, including coefficients, via direct calculation.http://www.phy.uct.ac.za/courses/phy300w/np/ch1/node38.html This calculation was first performed by physicist George Gamow
in 1928.
Nuclear physics
Nuclear physics is the field of physics that studies the building blocks and interactions of atomic nuclei. The most commonly known applications of nuclear physics are nuclear power generation and nuclear weapons technology, but the research has provided application in many fields, including those...
, the Geiger–Nuttall law or Geiger–Nuttall rule relates the decay constant of a radioactive isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
with the energy of the alpha particles emitted. Roughly speaking, it states that short-lived isotopes emit more energetic alpha particles than long-lived ones.
The relationship also shows that half-lives are exponentially dependent on decay energy, so that very large changes in half-life make comparatively small differences in decay energy, and thus alpha particle energy. In practice, this means that alpha particles from all alpha-emitting isotopes across many orders of magnitude of difference in half-life, all nevertheless have about the same decay energy.
Formulated in 1911 by Hans Geiger and John Mitchell Nuttall
John Mitchell Nuttall
John Mitchell Nuttall was an English physicist, born in Todmorden. He is best remembered for his work with the physicist Hans Geiger, which resulted in the Geiger-Nuttall law of radioactive decay....
, in its modern form the Geiger–Nuttall law is
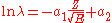
where λ is the decay constant (λ = ln2/half-life), Z the atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
, E the total kinetic energy
Kinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
(of the alpha particle and the daughter nucleus), and a1 and a2 are constant
Constant (mathematics)
In mathematics, a constant is a non-varying value, i.e. completely fixed or fixed in the context of use. The term usually occurs in opposition to variable In mathematics, a constant is a non-varying value, i.e. completely fixed or fixed in the context of use. The term usually occurs in opposition...
s.
Derivation
A simple way to derive this law is to consider an alpha particleAlpha particle
Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is classically produced in the process of alpha decay, but may be produced also in other ways and given the same name...
in the atomic nucleus as a particle in a box
Particle in a box
In quantum mechanics, the particle in a box model describes a particle free to move in a small space surrounded by impenetrable barriers. The model is mainly used as a hypothetical example to illustrate the differences between classical and quantum systems...
. The particle is in a bound state
Bound state
In physics, a bound state describes a system where a particle is subject to a potential such that the particle has a tendency to remain localised in one or more regions of space...
because of the presence of the strong interaction
Strong interaction
In particle physics, the strong interaction is one of the four fundamental interactions of nature, the others being electromagnetism, the weak interaction and gravitation. As with the other fundamental interactions, it is a non-contact force...
potential. It will constantly bounce from one side to the other, and due to the possibility of quantum tunneling by the wave though the potential barrier, each time it bounces, there will be a small likelihood for it to escape.
A knowledge of this quantum mechanical effect enables one to obtain this law, including coefficients, via direct calculation.http://www.phy.uct.ac.za/courses/phy300w/np/ch1/node38.html This calculation was first performed by physicist George Gamow
George Gamow
George Gamow , born Georgiy Antonovich Gamov , was a Russian-born theoretical physicist and cosmologist. He discovered alpha decay via quantum tunneling and worked on radioactive decay of the atomic nucleus, star formation, stellar nucleosynthesis, Big Bang nucleosynthesis, cosmic microwave...
in 1928.

