
Fluorine perchlorate
Encyclopedia
Fluorine perchlorate is the rarely encountered chemical compound
of fluorine
, chlorine
, and oxygen
with chemical formula
or . It is an extremely unstable gas that explodes spontaneously, and has a penetrating odor.
, though the action of ClF5
on water is another method.
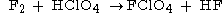
A third method of synthesis involves the thermal decomposition of tetrafluoroammonium
perchlorate, , which yields very pure that may be manipulated and frozen without explosions.
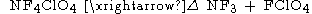
of 0, due to the electronegativity
of oxygen, which is higher than that of chlorine but lower than that of fluorine.
compounds, and other hazardous substances.
Accidental synthesis is possible if precursors are carelessly mixed. Like similar covalent fluorides and perchlorates, it needs to be handled with extreme caution.
ion:
FClO4 can also reacts with tetrafluoroethylene
:
It may be a radical
addition.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
of fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
, chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
with chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
or . It is an extremely unstable gas that explodes spontaneously, and has a penetrating odor.
Synthesis
One synthesis uses fluorine and perchloric acidPerchloric acid
Perchloric acid is the inorganic compound with the formula HClO4. Usually encountered as an aqueous solution, this colourless compound is a strong acid comparable in strength to sulfuric and nitric acids. It is a powerful oxidizer, but its aqueous solutions up to appr. 70% are remarkably inert,...
, though the action of ClF5
Chlorine pentafluoride
Chlorine pentafluoride is an interhalogen compound with formula ClF5. It was first synthesized in 1963.Its square pyramidal structure with C4v symmetry was confirmed by its high resolution19F NMR spectrum.-Preparation:...
on water is another method.
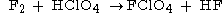
A third method of synthesis involves the thermal decomposition of tetrafluoroammonium
Tetrafluoroammonium
The tetrafluoroammonium cation is a positively-charged polyatomic ion with chemical formula . It is equivalent to the ammonium ion where the hydrogen atoms surrounding the central nitrogen atom have been replaced by fluorine....
perchlorate, , which yields very pure that may be manipulated and frozen without explosions.
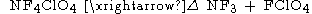
Structure
Fluorine perchlorate is not analogous to perchloric acid because the fluorine atom does not exist as a positive ion. It contains an oxygen atom in a rare oxidation stateOxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
of 0, due to the electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
of oxygen, which is higher than that of chlorine but lower than that of fluorine.
Safety
FClO4 has a very dangerous and unpredictable series of reactions associated with it, as a covalent perchlorate (chlorine in the +7 oxidation state) and a compound featuring a very sensitive O-F single bond. Small amounts of reducing agent, such as organic compounds, can trigger explosive detonation. Products of these decomposition reactions could include oxygen halides, interhalogenInterhalogen
The halogens react with each other to form interhalogen compounds.The general formula of most interhalogen compounds is XYn, where n = 1, 3, 5 or 7, and X is the less electronegative of the two halogens...
compounds, and other hazardous substances.
Accidental synthesis is possible if precursors are carelessly mixed. Like similar covalent fluorides and perchlorates, it needs to be handled with extreme caution.
Reaction
FClO4 is a strong oxidant and it can reacts with iodideIodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. This page is for the iodide ion and its salts. For information on organoiodides, see organohalides. In everyday life, iodide is most commonly encountered as a component of iodized salt,...
ion:
- FOClO3 + 2I- → ClO4- + F- + I2
FClO4 can also reacts with tetrafluoroethylene
Tetrafluoroethylene
Tetrafluoroethylene is a chemical compound with the formula C2F4. It is the simplest alkene fluorocarbon. This gaseous species is used primarily in the industrial preparation of polymers.-Properties:...
:
- CF2=CF2 + FOClO3 → CF3CF2OClO3
It may be a radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
addition.
External links
- Chemist Derek LoweDerek Lowe (chemist)Derek Lowe is a chemistry blogger and medicinal chemist working on preclinical drug discovery. Lowe got his BA from Hendrix College and his PhD in organic chemistry from Duke University, doing his doctoral research studying the organic synthesis of natural products, before spending time in Germany...
's experiences with perchlorates

