ERM protein family
Encyclopedia
The ERM protein family consists of three closely related protein
s, ezrin, radixin
and moesin
. The three paralogs, ezrin, radixin and moesin, are present in vertebrates whereas other species have only one ERM gene. Therefore, in vertebrates these paralogs likely arose by gene duplication.
ERM proteins are highly conserved throughout evolution . More than 75% identity is observed in the N-terminal and the C-terminal of vertebrates (ezrin, radixin, moesin), Drosophila
(dmoesin) and C. elegans
(ERM-1) homologues.
:
Ezrin, radixin and merlin also contain a polyproline
region between the central helical and C-terminal domains.
filaments with plasma membranes. They co-localize with CD44
at actin filament-plasma membrane interaction sites, associating with CD44 via their N-terminal domains and with actin filaments via their C-terminal domains.
In culture cells, ERM proteins mainly exhibit the folded conformation (about 80-85%).
The current model for ERM proteins activation is a two-steps mechanism:
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s, ezrin, radixin
Radixin
Radixin is a protein that in humans is encoded by the RDX gene.Radixin is a cytoskeletal protein that may be important in linking actin to the plasma membrane. It is highly similar in sequence to both ezrin and moesin. The radixin gene has been localized by fluorescence in situ hybridization to...
and moesin
Moesin
Moesin is a protein that in humans is encoded by the MSN gene.Moesin is a member of the ERM protein family which includes ezrin and radixin. ERM proteins appear to function as cross-linkers between plasma membranes and actin-based cytoskeletons...
. The three paralogs, ezrin, radixin and moesin, are present in vertebrates whereas other species have only one ERM gene. Therefore, in vertebrates these paralogs likely arose by gene duplication.
ERM proteins are highly conserved throughout evolution . More than 75% identity is observed in the N-terminal and the C-terminal of vertebrates (ezrin, radixin, moesin), Drosophila
Drosophila
Drosophila is a genus of small flies, belonging to the family Drosophilidae, whose members are often called "fruit flies" or more appropriately pomace flies, vinegar flies, or wine flies, a reference to the characteristic of many species to linger around overripe or rotting fruit...
(dmoesin) and C. elegans
Caenorhabditis elegans
Caenorhabditis elegans is a free-living, transparent nematode , about 1 mm in length, which lives in temperate soil environments. Research into the molecular and developmental biology of C. elegans was begun in 1974 by Sydney Brenner and it has since been used extensively as a model...
(ERM-1) homologues.
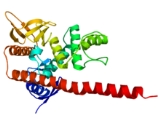
Structure
ERM molecules contain the following three domainsProtein domain
A protein domain is a part of protein sequence and structure that can evolve, function, and exist independently of the rest of the protein chain. Each domain forms a compact three-dimensional structure and often can be independently stable and folded. Many proteins consist of several structural...
:
- N-terminal globular domain, also called FERM domainFERM domainIn molecular biology, the FERM domain is a widespread protein module involved in localising proteins to the plasma membrane. FERM domains are found in a number of cytoskeletal-associated proteins that associate with various proteins at the interface between the plasma membrane and the cytoskeleton...
(Band 4.1Band 4.1Protein 4.1, also known as Beatty's Protein, is a protein associated with the cytoskeleton that in humans is encoded by the EPB41 gene.Protein 4.1 is a major structural element of the erythrocyte membrane skeleton. It plays a key role in regulating membrane physical properties of mechanical...
, ezrin, radixinRadixinRadixin is a protein that in humans is encoded by the RDX gene.Radixin is a cytoskeletal protein that may be important in linking actin to the plasma membrane. It is highly similar in sequence to both ezrin and moesin. The radixin gene has been localized by fluorescence in situ hybridization to...
, moesinMoesinMoesin is a protein that in humans is encoded by the MSN gene.Moesin is a member of the ERM protein family which includes ezrin and radixin. ERM proteins appear to function as cross-linkers between plasma membranes and actin-based cytoskeletons...
). The FERM domain allows ERM proteins to interact with integral proteins of the plasma membrane, or scaffolding proteins localized beneath the plasma membrane. The FERM domain is composed of three subdomains (F1, F2, F3) that are arranged as a cloverleaf. - extended alpha-helicalAlpha helixA common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
domain. - charged C-terminal domain. This domain mediates the interaction with F-actin.
Ezrin, radixin and merlin also contain a polyproline
Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
region between the central helical and C-terminal domains.
Function
ERM proteins crosslink actinActin
Actin is a globular, roughly 42-kDa moonlighting protein found in all eukaryotic cells where it may be present at concentrations of over 100 μM. It is also one of the most highly-conserved proteins, differing by no more than 20% in species as diverse as algae and humans...
filaments with plasma membranes. They co-localize with CD44
CD44
The CD44 antigen is a cell-surface glycoprotein involved in cell–cell interactions, cell adhesion and migration. In humans, the CD44 antigen is encoded by the CD44 gene.- Tissue distribution and isoforms :...
at actin filament-plasma membrane interaction sites, associating with CD44 via their N-terminal domains and with actin filaments via their C-terminal domains.
Activation
ERM proteins are highly regulated proteins. They exist in two forms:- the FERM domain is able to interact with the F-actin binding site and this head-to-tail interaction maintains ERM proteins into a folded form; in this state, ERM proteins are inactive for the folding prevents either integral protein binding, or actin-binding.
- if this head-to-tail interaction is disrupted, ERM proteins unfold, leading to an open and active conformation.
In culture cells, ERM proteins mainly exhibit the folded conformation (about 80-85%).
The current model for ERM proteins activation is a two-steps mechanism:
- First, phosphatidylinositol 4,5-bisphosphatePhosphatidylinositol 4,5-bisphosphatePhosphatidylinositol 4,5-bisphosphate or PtdInsP2, also known simply as PIP2, is a minor phospholipid component of cell membranes...
interaction at the plasma membrane induces a pre-opening of ERM molecule - Then, a not yet identified kinase phosphorylates a Threonine localized in a highly conserved region of the C-terminal domain. The phosphate will stabilize the opening of the molecule

