
DNA glycosylases
Encyclopedia
DNA glycosylases are a family of enzyme
s involved in base excision repair
, classified under EC number
EC 3.2.2. Base excision repair
is the mechanism by which damaged bases in DNA
are removed and replaced. DNA glycosylases catalyze the first step of this process. They remove the damaged nitrogenous base while leaving the sugar-phosphate backbone intact, creating an apurinic/apyrimidinic site, commonly referred to as an AP site
. This is accomplished by flipping the damaged base out of the double helix followed by cleavage of the N-glycosidic bond. Glycosylases were first discovered in bacteria, and have since been found in all kingdoms of life. In addition to their role in base excision repair DNA glycosylase enzymes have been implicated in the repression of gene silencing in A. thaliana, N. tabacum and other plants by active demethylation. 5-methylcytosine residues are excised and replaced with unmethylated cytosines allowing acces to the chromatin structure of the enzymes and proteins necessary for trancription and subsequent translation.
activity that permits them to cut the phosphodiester bond
of DNA, creating a single-strand break without the need for an AP endonuclease. β-Elimination of an AP site by a glycosylase-lyase yields a 3' α,β-unsaturated aldehyde adjacent to a 5' phosphate, which differs from the AP endonuclease cleavage product. Some glycosylase-lyases can further perform δ-elimination, which converts the 3' aldehyde to a 3' phosphate.
of a DNA glycosylase was obtained for E. coli Nth. This structure revealed that the enzyme flips the damaged base out of the double helix into an active site pocket in order to excise it. Other glycosylases have since been found to follow the same general paradigm, including human UNG pictured below. To cleave the N-glycosidic bond, monofunctional glycosylases use an activated water molecule to attack carbon 1 of the substrate. Bifunctional glycosylases, instead, use an amine residue as a nucleophile to attack the same carbon, going through a Schiff base
intermediate.
of many glycosylases have been solved. Based on structural similarity, glycosylases are grouped into four superfamilies. The UDG and AAG families contain small, compact glycosylases, whereas the MutM/Fpg and HhH-GPD families comprise larger enzymes with multiple domains.
A wide variety of glycosylases have evolved to recognize different damaged bases. The table below summarizes the properties of known glycosylases in commonly studied model organisms.
DNA glycosylases can be grouped into the following categories based on their substrate(s):
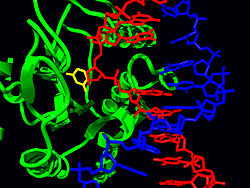 Uracil DNA glycosylases remove uracil
Uracil DNA glycosylases remove uracil
from DNA, which can arise either by spontaneous deamination of cytosine or by the misincorporation of dU opposite dA during DNA replication
. The prototypical member of this family is E. coli UDG, which was among the first glycosylases discovered. Four different uracil-DNA glycosylase activities have been identified in mammalian cells, including UNG, SMUG1
, TDG
, and MBD4
. They vary in substrate specificity and subcellular localization. SMUG1 prefers single-stranded DNA as substrate, but also removes U from double-stranded DNA. In addition to unmodified uracil, SMUG1 can excise 5-hydroxyuracil, 5-hydroxymethyluracil and 5-formyluracil bearing an oxidized group at ring C5. TDG and MBD4 are strictly specific for double-stranded DNA. TDG can remove thymine glycol when present opposite guanine, as well as derivatives of U with modifications at carbon 5. Current evidence suggests that, in human cells, TDG and SMUG1 are the major enzymes responsible for the repair of the U:G mispairs caused by spontaneous cytosine deamination, whereas uracil arising in DNA through dU misincorporation is mainly dealt with by UNG. MBD4 is thought to correct T:G mismatches that arise from deamination of 5-methylcytosine to thymine in CpG sites. MBD4 mutant mice develop normally and do not show increased cancer susceptibility or reduced survival. But they acquire more C T mutations at CpG sequences in epithelial cells of the small intestine.
The structure of human UNG in complex with DNA revealed that, like other glycosylases, it flips the target nucleotide out of the double helix and into the active site pocket. UDG undergoes a conformational change from an ‘‘open’’ unbound state to a ‘‘closed’’ DNA-bound state.
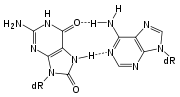 A variety of glycosylases have evolved to recognize oxidized bases, which are commonly formed by reactive oxygen species generated during cellular metabolism. The most abundant lesions formed at guanine residues are 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FapyG) and 8-oxoguanine
A variety of glycosylases have evolved to recognize oxidized bases, which are commonly formed by reactive oxygen species generated during cellular metabolism. The most abundant lesions formed at guanine residues are 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FapyG) and 8-oxoguanine
. Due to mispairing with adenine during replication, 8-oxoG is highly mutagenic, resulting in G to T transversions. Repair of this lesion is initiated by the bifunctional DNA glycosylase OGG1, which recognizes 8-oxoG paired with C.
hOGG1 is a bifunctional glycosylase that belongs to the helix-hairpin-helix (HhH) family. MYH
recognizes adenine mispaired with 8-oxoG but excises the A, leaving the 8-oxoG intact. OGG1 knockout mice do not show an increased tumor incidence, but accumulate 8-oxoG in the liver as they age. A similar phenotype is observed with the inactivation of MYH, but simultaneous inactivation of both MYH and OGG1 causes 8-oxoG accumulation in multiple tissues including lung and small intestine. In humans, mutations in MYH are associated with increased risk of developing colon polyps
and colon cancer. In addition to OGG1 and MYH, human cells contain three additional DNA glycosylases, NEIL1
, NEIL2
, and NEIL3. These are homologous to bacterial Nei, and their presence likely explains the mild phenotypes of the OGG1 and MYH knockout mice.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s involved in base excision repair
Base excision repair
In biochemistry and genetics, base excision repair is a cellular mechanism that repairs damaged DNA throughout the cell cycle. It is responsible primarily for removing small, non-helix-distorting base lesions from the genome. The related nucleotide excision repair pathway repairs bulky...
, classified under EC number
EC number
The Enzyme Commission number is a numerical classification scheme for enzymes, based on the chemical reactions they catalyze....
EC 3.2.2. Base excision repair
Base excision repair
In biochemistry and genetics, base excision repair is a cellular mechanism that repairs damaged DNA throughout the cell cycle. It is responsible primarily for removing small, non-helix-distorting base lesions from the genome. The related nucleotide excision repair pathway repairs bulky...
is the mechanism by which damaged bases in DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
are removed and replaced. DNA glycosylases catalyze the first step of this process. They remove the damaged nitrogenous base while leaving the sugar-phosphate backbone intact, creating an apurinic/apyrimidinic site, commonly referred to as an AP site
AP site
In biochemistry and molecular genetics, an AP site , also known as an abasic site, is a location in DNA that has neither a purine nor a pyrimidine base, either spontaneously or due to DNA damage...
. This is accomplished by flipping the damaged base out of the double helix followed by cleavage of the N-glycosidic bond. Glycosylases were first discovered in bacteria, and have since been found in all kingdoms of life. In addition to their role in base excision repair DNA glycosylase enzymes have been implicated in the repression of gene silencing in A. thaliana, N. tabacum and other plants by active demethylation. 5-methylcytosine residues are excised and replaced with unmethylated cytosines allowing acces to the chromatin structure of the enzymes and proteins necessary for trancription and subsequent translation.
Monofunctional vs. bifunctional glycosylases
There are two main classes of glycosylases: monofunctional and bifunctional. Monofunctional glycosylases have only glycosylase activity, whereas bifunctional glycosylases also possess AP lyaseLyase
In biochemistry, a lyase is an enzyme that catalyzes the breaking of various chemical bonds by means other than hydrolysis and oxidation, often forming a new double bond or a new ring structure...
activity that permits them to cut the phosphodiester bond
Phosphodiester bond
A phosphodiester bond is a group of strong covalent bonds between a phosphate group and two 5-carbon ring carbohydrates over two ester bonds. Phosphodiester bonds are central to all known life, as they make up the backbone of each helical strand of DNA...
of DNA, creating a single-strand break without the need for an AP endonuclease. β-Elimination of an AP site by a glycosylase-lyase yields a 3' α,β-unsaturated aldehyde adjacent to a 5' phosphate, which differs from the AP endonuclease cleavage product. Some glycosylase-lyases can further perform δ-elimination, which converts the 3' aldehyde to a 3' phosphate.
Biochemical mechanism
The first crystal structureCrystallography
Crystallography is the experimental science of the arrangement of atoms in solids. The word "crystallography" derives from the Greek words crystallon = cold drop / frozen drop, with its meaning extending to all solids with some degree of transparency, and grapho = write.Before the development of...
of a DNA glycosylase was obtained for E. coli Nth. This structure revealed that the enzyme flips the damaged base out of the double helix into an active site pocket in order to excise it. Other glycosylases have since been found to follow the same general paradigm, including human UNG pictured below. To cleave the N-glycosidic bond, monofunctional glycosylases use an activated water molecule to attack carbon 1 of the substrate. Bifunctional glycosylases, instead, use an amine residue as a nucleophile to attack the same carbon, going through a Schiff base
Schiff base
A Schiff base, named after Hugo Schiff, is a compound with a functional group that contains a carbon-nitrogen double bond with the nitrogen atom connected to an aryl or alkyl group, not hydrogen....
intermediate.
Types of glycosylases
Crystal structuresCrystallography
Crystallography is the experimental science of the arrangement of atoms in solids. The word "crystallography" derives from the Greek words crystallon = cold drop / frozen drop, with its meaning extending to all solids with some degree of transparency, and grapho = write.Before the development of...
of many glycosylases have been solved. Based on structural similarity, glycosylases are grouped into four superfamilies. The UDG and AAG families contain small, compact glycosylases, whereas the MutM/Fpg and HhH-GPD families comprise larger enzymes with multiple domains.
A wide variety of glycosylases have evolved to recognize different damaged bases. The table below summarizes the properties of known glycosylases in commonly studied model organisms.
| E. coli | Yeast (S. cerevisiae) Saccharomyces cerevisiae Saccharomyces cerevisiae is a species of yeast. It is perhaps the most useful yeast, having been instrumental to baking and brewing since ancient times. It is believed that it was originally isolated from the skin of grapes... |
Human | Type | Substrates |
|---|---|---|---|---|
| AlkA | Mag1 | MPG | monofunctional | 3-meA, hypoxanthine |
| UDG | Ung1 | UNG | monofunctional | uracil |
| Fpg | Ogg1 | hOGG1 | bifunctional | 8-oxoG, FapyG |
| Nth | Ntg1 | hNTH1 | bifunctional | Tg, hoU, hoC, urea, FapyG |
| Ntg2 | ||||
| Nei | Not present | hNEIL1 | bifunctional | Tg, hoU, hoC, urea, FapyG, FapyA |
| hNEIL2 | AP site, hoU | |||
| hNEIL3 | unknown | |||
| MutY | Not present | hMYH | monofunctional | A:8-oxoG |
| Not present | Not present | hSMUG1 | monofunctional | U, hoU, hmU, fU |
| Not present | Not present | TDG | monofunctional | T:G mispair |
| Not present | Not present | MBD4 | monofunctional | T:G mispair |
DNA glycosylases can be grouped into the following categories based on their substrate(s):
Uracil DNA glycosylases

Uracil
Uracil is one of the four nucleobases in the nucleic acid of RNA that are represented by the letters A, G, C and U. The others are adenine, cytosine, and guanine. In RNA, uracil binds to adenine via two hydrogen bonds. In DNA, the uracil nucleobase is replaced by thymine.Uracil is a common and...
from DNA, which can arise either by spontaneous deamination of cytosine or by the misincorporation of dU opposite dA during DNA replication
DNA replication
DNA replication is a biological process that occurs in all living organisms and copies their DNA; it is the basis for biological inheritance. The process starts with one double-stranded DNA molecule and produces two identical copies of the molecule...
. The prototypical member of this family is E. coli UDG, which was among the first glycosylases discovered. Four different uracil-DNA glycosylase activities have been identified in mammalian cells, including UNG, SMUG1
SMUG1
Single-strand selective monofunctional uracil DNA glycosylase is an enzyme that in humans is encoded by the SMUG1 gene.-Further reading:...
, TDG
Thymine-DNA glycosylase
G/T mismatch-specific thymine DNA glycosylase is an enzyme that in humans is encoded by the TDG gene.-Interactions:Thymine-DNA glycosylase has been shown to interact with Estrogen receptor alpha, SUMO3, CREB-binding protein, Promyelocytic leukemia protein and Small ubiquitin-related modifier...
, and MBD4
MBD4
Methyl-CpG-binding domain protein 4 is a protein that in humans is encoded by the MBD4 gene.-Interactions:MBD4 has been shown to interact with MLH1 and FADD.-Further reading:...
. They vary in substrate specificity and subcellular localization. SMUG1 prefers single-stranded DNA as substrate, but also removes U from double-stranded DNA. In addition to unmodified uracil, SMUG1 can excise 5-hydroxyuracil, 5-hydroxymethyluracil and 5-formyluracil bearing an oxidized group at ring C5. TDG and MBD4 are strictly specific for double-stranded DNA. TDG can remove thymine glycol when present opposite guanine, as well as derivatives of U with modifications at carbon 5. Current evidence suggests that, in human cells, TDG and SMUG1 are the major enzymes responsible for the repair of the U:G mispairs caused by spontaneous cytosine deamination, whereas uracil arising in DNA through dU misincorporation is mainly dealt with by UNG. MBD4 is thought to correct T:G mismatches that arise from deamination of 5-methylcytosine to thymine in CpG sites. MBD4 mutant mice develop normally and do not show increased cancer susceptibility or reduced survival. But they acquire more C T mutations at CpG sequences in epithelial cells of the small intestine.
The structure of human UNG in complex with DNA revealed that, like other glycosylases, it flips the target nucleotide out of the double helix and into the active site pocket. UDG undergoes a conformational change from an ‘‘open’’ unbound state to a ‘‘closed’’ DNA-bound state.
Glycosylases of oxidized bases

8-oxoguanine
8-Oxoguanine is one of the most common DNA lesions resulting from reactive oxygen species and can result in a mismatched pairing with Adenine resulting in G to T and C to A substitutions in the genome. In humans, it is primarily repaired by the DNA glycosylase OGG1...
. Due to mispairing with adenine during replication, 8-oxoG is highly mutagenic, resulting in G to T transversions. Repair of this lesion is initiated by the bifunctional DNA glycosylase OGG1, which recognizes 8-oxoG paired with C.
hOGG1 is a bifunctional glycosylase that belongs to the helix-hairpin-helix (HhH) family. MYH
MUTYH
MUTYH is a human gene encoding a DNA glycosylase, MUTYH glycosylase, involved in oxidative DNA damage repair. The enzyme excises adenine bases from the DNA backbone at sites where adenine is inappropriately paired with guanine, cytosine, or 8-oxo-7,8-dihydroguanine, a major oxidatively damaged DNA...
recognizes adenine mispaired with 8-oxoG but excises the A, leaving the 8-oxoG intact. OGG1 knockout mice do not show an increased tumor incidence, but accumulate 8-oxoG in the liver as they age. A similar phenotype is observed with the inactivation of MYH, but simultaneous inactivation of both MYH and OGG1 causes 8-oxoG accumulation in multiple tissues including lung and small intestine. In humans, mutations in MYH are associated with increased risk of developing colon polyps
Polyp (medicine)
A polyp is an abnormal growth of tissue projecting from a mucous membrane. If it is attached to the surface by a narrow elongated stalk, it is said to be pedunculated. If no stalk is present, it is said to be sessile. Polyps are commonly found in the colon, stomach, nose, sinus, urinary bladder...
and colon cancer. In addition to OGG1 and MYH, human cells contain three additional DNA glycosylases, NEIL1
NEIL1
Endonuclease VIII-like 1 is an enzyme that in humans is encoded by the NEIL1 gene.-Further reading:...
, NEIL2
NEIL2
Endonuclease VIII-like 2 is an enzyme that in humans is encoded by the NEIL2 gene.-Further reading:...
, and NEIL3. These are homologous to bacterial Nei, and their presence likely explains the mild phenotypes of the OGG1 and MYH knockout mice.

