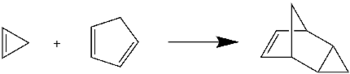Cyclopropene
Encyclopedia
Cyclopropene is an organic compound
with the formula
34. It is the simplest isolable cycloalkene
. It has a triangular
structure. Because the ring is highly strained
, cyclopropene is both difficult to prepare and interesting to study. Like cyclopropane
, the carbon ring of cyclopropene is planar. The reduced length of the double bond
compared to an single bond
causes the angle opposite the double bond to narrow to about 51° from the 60° angle found in cyclopropane. As with cyclopropane, the carbon–carbon bonding in the ring has increased p character
: the alkene carbons use sp2.68 hybridization
for the ring.
of trimethylcyclopropylammonium hydroxide over platinized clay at 320–330 °C under a CO2 atmosphere. This reaction produces mainly trimethylamine
and dimethylcyclopropyl amine, together with about 5% of cyclopropene. Cyclopropene can also be obtained in about 1% yield by thermolysis of the adduct of cycloheptatriene
and dimethyl acetylenedicarboxylate
.
undergoes dehydrohalogenation
upon treatment with the base sodium amide
at 80 °C to produce cyclopropene in about 10% yield.
The major byproduct of the reaction is allyl amine. Adding allyl chloride to sodium bis(trimethylsilyl)amide
in boiling toluene
over a period of 45–60 minutes produces the targeted compound in about 40% yield with an improvement in purity:
1-Methylcyclopropene is synthesized similarly but at room temperature from methallylchloride using phenyllithium
as the base:
eliminates the nitrite, giving the respective cyclopropene derivative. The synthesis of purely aliphatic cyclopropenes was first illustrated by the copper-catalyzed additions of carbenes to alkynes. In the presence of a copper sulfate catalyst, ethyl diazoacetate
reacts with acetylenes to give cyclopropenes. 1,2-Dimethylcyclopropene-3-carboxylate arises via this method from 2-butyne. Copper has proved to be useful as a catalyst in a variety of cyclopropene syntheses. Copper sulfate and copper dust are among the more popular forms of copper used.
(propyne).
Attempted fractional distillation of cyclopropene at –36 °C (its predicted boiling point) results in polymerization. The mechanism is assumed to be a free-radical chain reaction, and the product, based on NMR spectra, is thought to be polycyclopropane.
Cyclopropene undergoes the Diels–Alder reaction with cyclopentadiene
to give endo-tricyclo[3.2.1.02,4]oct-6-ene. This reaction is commonly used to check for the presence of cyclopropene, following its synthesis.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
with the formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
34. It is the simplest isolable cycloalkene
Cycloalkene
A cycloalkene or cycloolefin is a type of alkene hydrocarbon which contains a closed ring of carbon atoms, but has no aromatic character. Some cycloalkenes, such as cyclobutene and cyclopentene, can be used as monomers to produce polymer chains. Unless the rings are very large, cycloalkenes are...
. It has a triangular
Triangle
A triangle is one of the basic shapes of geometry: a polygon with three corners or vertices and three sides or edges which are line segments. A triangle with vertices A, B, and C is denoted ....
structure. Because the ring is highly strained
Ring strain
In organic chemistry, ring strain is the tendency of a cyclic molecule, such as cyclopropane, to destabilize when its atoms are in non-favorable high energy spatial orientations...
, cyclopropene is both difficult to prepare and interesting to study. Like cyclopropane
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
, the carbon ring of cyclopropene is planar. The reduced length of the double bond
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
compared to an single bond
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
causes the angle opposite the double bond to narrow to about 51° from the 60° angle found in cyclopropane. As with cyclopropane, the carbon–carbon bonding in the ring has increased p character
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
: the alkene carbons use sp2.68 hybridization
Orbital hybridisation
In chemistry, hybridisation is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties. Hybridised orbitals are very useful in the explanation of the shape of molecular orbitals for molecules. It is an integral part...
for the ring.
Early syntheses
The first confirmed synthesis of cyclopropene, carried out by Dem'yanov and Doyarenko, involved the thermal decompositionThermal decomposition
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes....
of trimethylcyclopropylammonium hydroxide over platinized clay at 320–330 °C under a CO2 atmosphere. This reaction produces mainly trimethylamine
Trimethylamine
Trimethylamine is an organic compound with the formula N3. This colorless, hygroscopic, and flammable tertiary amine has a strong "fishy" odor in low concentrations and an ammonia-like odor at higher concentrations...
and dimethylcyclopropyl amine, together with about 5% of cyclopropene. Cyclopropene can also be obtained in about 1% yield by thermolysis of the adduct of cycloheptatriene
Cycloheptatriene
Cycloheptatriene is an organic compound with the formula C7H8. This colourless liquid has been of recurring theoretical interest in organic chemistry. It is a ligand in organometallic chemistry and as a building block in organic synthesis...
and dimethyl acetylenedicarboxylate
Dimethyl acetylenedicarboxylate
Dimethyl acetylenedicarboxylate is the organic compound with the formula CH3O2CC2CO2CH3. This ester, which exists as a liquid at room temperature, is highly electrophilic. As such, DMAD, as it is commonly called in the laboratory, is widely employed as a dienophile in cycloaddition reactions,...
.
Modern syntheses from allyl chlorides
Allyl chlorideAllyl chloride
Allyl chloride is the organic compound with the formula CH2=CHCH2Cl. This colorless liquid is insoluble in water but soluble in common organic solvents. It is mainly converted to epichlorohydrin, used in the production of plastics. It is a chlorinated derivative of propylene.-Production:Allyl...
undergoes dehydrohalogenation
Dehydrohalogenation
Dehydrohalogenation is an organic reaction from which an alkene is obtained from an alkyl halide . It is also called a β-Elimination reaction and is a type of elimination reaction....
upon treatment with the base sodium amide
Sodium amide
Sodium amide, commonly called sodamide, is the chemical compound with the formula NaNH2. This solid, which is dangerously reactive toward water, is white when pure, but commercial samples are typically gray due to the presence of small quantities of metallic iron from the manufacturing process...
at 80 °C to produce cyclopropene in about 10% yield.
-
- CH2=CHCH2Cl + NaNH2 → C3H4 (cyclopropene) + NaCl + NH3
The major byproduct of the reaction is allyl amine. Adding allyl chloride to sodium bis(trimethylsilyl)amide
Sodium bis(trimethylsilyl)amide
Sodium bisamide is the chemical compound with the formula 2NNa. This species, usually called NaHMDS , is a strong base used for deprotonation reactions or base catalyzed reaction...
in boiling toluene
Toluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
over a period of 45–60 minutes produces the targeted compound in about 40% yield with an improvement in purity:
-
- CH2=CHCH2Cl + NaN(TMS)2 → C3H4 (cyclopropene) + NaCl + NH(TMS)2
1-Methylcyclopropene is synthesized similarly but at room temperature from methallylchloride using phenyllithium
Phenyllithium
Phenyllithium is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses...
as the base:
-
- CH2=C(CH3)CH2Cl + LiC6H5 → CH3C3H3 (1-methylcylopropene) + LiCl + C6H6
Syntheses of derivatives
Treatment of nitrocyclopropanes with sodium methoxideSodium methoxide
Sodium methoxide is a chemical compound, with formula CH3ONa. This colourless solid, which is formed by the deprotonation of methanol, is a widely used reagent in industry and the laboratory...
eliminates the nitrite, giving the respective cyclopropene derivative. The synthesis of purely aliphatic cyclopropenes was first illustrated by the copper-catalyzed additions of carbenes to alkynes. In the presence of a copper sulfate catalyst, ethyl diazoacetate
Ethyl diazoacetate
Ethyl diazoacetate is a diazo compound and a reagent in organic chemistry. It was discovered by Theodor Curtius in 1883. The compound can be prepared by reaction of the ethyl ester of glycine with sodium nitrite and sodium acetate in water....
reacts with acetylenes to give cyclopropenes. 1,2-Dimethylcyclopropene-3-carboxylate arises via this method from 2-butyne. Copper has proved to be useful as a catalyst in a variety of cyclopropene syntheses. Copper sulfate and copper dust are among the more popular forms of copper used.
Reactions of cyclopropene
Studies on cyclopropene mainly focus on the consequences of its high ring strain. At 425 °C, cyclopropene isomerizes to methylacetyleneMethylacetylene
Methylacetylene is an alkyne with the chemical formula H3C≡CH. It is a component of MAPP gas along with its isomer 1,2-propadiene , which is commonly used in gas welding...
(propyne).
- C3H4 → H3CC≡CH
Attempted fractional distillation of cyclopropene at –36 °C (its predicted boiling point) results in polymerization. The mechanism is assumed to be a free-radical chain reaction, and the product, based on NMR spectra, is thought to be polycyclopropane.
Cyclopropene undergoes the Diels–Alder reaction with cyclopentadiene
Cyclopentadiene
Cyclopentadiene is an organic compound with the formula C5H6. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction...
to give endo-tricyclo[3.2.1.02,4]oct-6-ene. This reaction is commonly used to check for the presence of cyclopropene, following its synthesis.
Related compounds
- Malvalic acidMalvalic acidMalvalic acid is a cyclopropenic fatty acid found in cottonseed oil. The cyclopropene ring is thought to be one of the causes of abnormalities that develop in animals that ingest cottonseed oil. This reactivity could be cause for concern depending on concentration. Hydrogenation of the oil destroys...
is a toxic cyclopropene fatty acid that occurs in cottonseed oil. - 1-Methylcyclopropene1-Methylcyclopropene1-Methylcyclopropene is a cyclopropene derivative used as a synthetic plant growth regulator. It is structurally related to the natural plant hormone ethylene and it is used commercially to slow down the ripening of fruit and to help maintain the freshness of cut flowers.-Chemical...
(1-MCP) is used to slow the ripening in fruits. - Borirenes, phosphirenes, and silirenes are boron-, phosphorus-, and silicon-substituted cyclopropenes, with the formula RBC2R'2, RPC2R'2, and R2SiC2R'2.