Cyclobutadieneiron tricarbonyl
Encyclopedia
Cyclobutadieneiron tricarbonyl or (C4H4)Fe(CO)3 is an organometallic complex of cyclobutadiene
and an iron
metal carbonyl
. The chemical compound
is used in organic chemistry
as a precursor for cyclobutadiene. It was first prepared in 1965 by Rowland Pettit starting from cyclooctatetraene
:
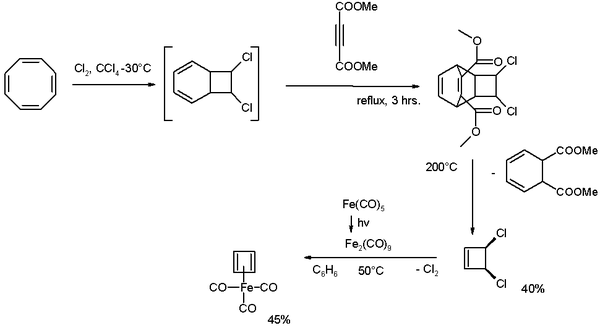
Cyclooctatetraene is chlorinated to the [4.2.0]-bicyclic compound which reacts further with the alkyne
dimethyl acetylenedicarboxylate
in a Diels-Alder reaction
followed by a reverse-DA reaction by pyrolysis
at 200°C releasing cis-dichlorocyclobutene.
This compound reacts with di-iron nonacarbonyl (obtained from photolysis of iron pentacarbonyl
) to Cyclobutadieneiron tricarbonyl.
Cyclobutadieneiron tricarbonyl displays aromaticity
as evidenced by some of its reactions which can be classified as electrophilic aromatic substitution
:
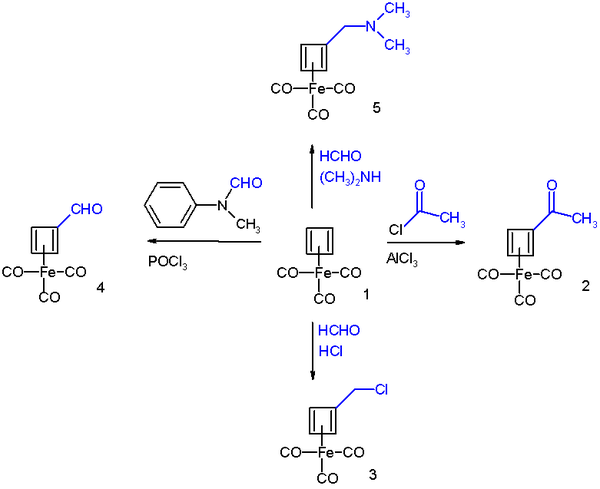
It reacts in Friedel-Crafts acylation with acetyl chloride and aluminium chloride to the acyl derivative 2, with formaldehyde
and hydrochloric acid
to the chloromethyl derivative 3, in a Vilsmeier-Haack reaction
with N-methylformanilide and phosphorus oxychloride to the formyl 4 and in a Mannich reaction
to amine derivative 4
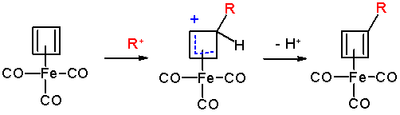
The reaction mechanism
is identical to that of EAS:
Cyclobutadiene
Cyclobutadiene is the smallest [n]-annulene , an extremely unstable hydrocarbon having a lifetime shorter than five seconds in the free state. It has chemical formula 44 and a rectangular structure verified by infrared studies. This is in contrast to the square geometry predicted by simple Hückel...
and an iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
metal carbonyl
Metal carbonyl
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. These complexes may be homoleptic, that is containing only CO ligands, such as nickel carbonyl , but more commonly metal carbonyls contain a mix of ligands, such as Re3Cl...
. The chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
is used in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
as a precursor for cyclobutadiene. It was first prepared in 1965 by Rowland Pettit starting from cyclooctatetraene
Cyclooctatetraene
1,3,5,7-Cyclooctatetraene is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature...
:
Cyclooctatetraene is chlorinated to the [4.2.0]-bicyclic compound which reacts further with the alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
dimethyl acetylenedicarboxylate
Dimethyl acetylenedicarboxylate
Dimethyl acetylenedicarboxylate is the organic compound with the formula CH3O2CC2CO2CH3. This ester, which exists as a liquid at room temperature, is highly electrophilic. As such, DMAD, as it is commonly called in the laboratory, is widely employed as a dienophile in cycloaddition reactions,...
in a Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
followed by a reverse-DA reaction by pyrolysis
Pyrolysis
Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase, and is irreversible...
at 200°C releasing cis-dichlorocyclobutene.
This compound reacts with di-iron nonacarbonyl (obtained from photolysis of iron pentacarbonyl
Iron pentacarbonyl
Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula 5. Under standard conditions Fe5 is a free-flowing, straw-colored liquid with a pungent odour. This compound is a common precursor to diverse iron compounds, including many that are useful in organic synthesis. Fe5 is...
) to Cyclobutadieneiron tricarbonyl.
Cyclobutadieneiron tricarbonyl displays aromaticity
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
as evidenced by some of its reactions which can be classified as electrophilic aromatic substitution
Electrophilic aromatic substitution
Electrophilic aromatic substitution EAS is an organic reaction in which an atom, usually hydrogen, appended to an aromatic system is replaced by an electrophile...
:
It reacts in Friedel-Crafts acylation with acetyl chloride and aluminium chloride to the acyl derivative 2, with formaldehyde
Formaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
and hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
to the chloromethyl derivative 3, in a Vilsmeier-Haack reaction
Vilsmeier-Haack reaction
The Vilsmeier–Haack reaction is the chemical reaction of a substituted amide with phosphorus oxychloride and an electron-rich arene to produce an aryl aldehyde or ketone . The reaction is named after Anton Vilsmeier and Albrecht Haack...
with N-methylformanilide and phosphorus oxychloride to the formyl 4 and in a Mannich reaction
Mannich reaction
The Mannich reaction is an organic reaction which consists of an amino alkylation of an acidic proton placed next to a carbonyl functional group with formaldehyde and ammonia or any primary or secondary amine. The final product is a β-amino-carbonyl compound also known as a Mannich base...
to amine derivative 4
The reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
is identical to that of EAS: