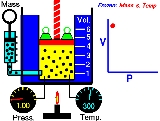
Boyle's law
Overview
Gas laws
The early gas laws were developed at the end of the 18th century, when scientists began to realize that relationships between the pressure, volume and temperature of a sample of gas could be obtained which would hold for all gases...
and a special case of the ideal gas law
Ideal gas law
The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law...
. Boyle's law describes the inversely proportional relationship between the absolute pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
and volume
Volume
Volume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
of a gas, if the temperature is kept constant within a closed system
Closed system
-In physics:In thermodynamics, a closed system can exchange energy , but not matter, with its surroundings.In contrast, an isolated system cannot exchange any of heat, work, or matter with the surroundings, while an open system can exchange all of heat, work and matter.For a simple system, with...
. The law was named after chemist
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
and physicist
Physicist
A physicist is a scientist who studies or practices physics. Physicists study a wide range of physical phenomena in many branches of physics spanning all length scales: from sub-atomic particles of which all ordinary matter is made to the behavior of the material Universe as a whole...
Robert Boyle
Robert Boyle
Robert Boyle FRS was a 17th century natural philosopher, chemist, physicist, and inventor, also noted for his writings in theology. He has been variously described as English, Irish, or Anglo-Irish, his father having come to Ireland from England during the time of the English plantations of...
, who published the original law in 1662. The law itself can be stated as follows:
This relationship between pressure and volume was first noted by two amateur scientists, Richard Towneley
Richard Towneley
Richard Towneley was an English mathematician and astronomer from Towneley near Burnley, Lancashire. He was one of a group of seventeenth century astronomers in the north of England, which included Jeremiah Horrocks, William Crabtree and William Gascoigne, the pioneer astronomers who laid the...
and Henry Power
Henry Power
Henry Power was an English physician and experimenter, one of the first elected Fellows of the Royal Society.-Life:He matriculated as a pensioner of Christ's College, Cambridge, in 1641, and graduated B.A. in 1644. He became a regular correspondent of Sir Thomas Browne on scientific subjects. He...
.

