Bisulfide
Encyclopedia
The bisulfide ion, also called hydrogensulfide or hydrosulfide, is the anion with the formula
[HS]− (also commonly written [SH]− by analogy to [OH]−). This species is the conjugate base of hydrogen sulfide
and in its turn also dissociates in sulfide
:
In aqueous solutions, at pH lower than 7, hydrogen sulfide (H2S) is the dominant species but at pH higher than 7, bisulfide (HS−) dominates. Sulfide (S2−) is an extremely basic group and only dominates in strongly alkaline conditions at elevated pH.
A variety of salts are known, including sodium hydrosulfide
and potassium hydrosulfide
. Ammonium hydrosulfide, a component of "stink bombs" has not been isolated as a pure solid. Some compounds described as salts of the sulfide
dianion contain primarily hydrosulfide. For example, the hydrated form of sodium sulfide
, nominally with the formula Na2S · 9 H2O, is better described as NaSH · NaOH · 8 H2O.
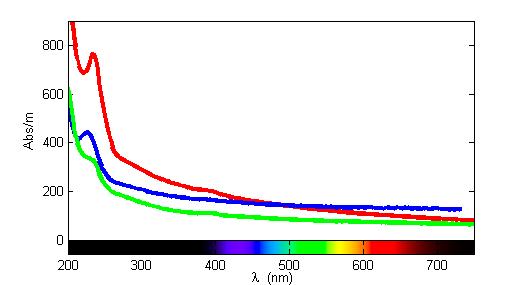 Aqueous bisulfide absorbs light at around 230 nm in the UV/VIS spectrum
Aqueous bisulfide absorbs light at around 230 nm in the UV/VIS spectrum
. Research groups have used field spectrometers to measure the absorption due to bisulfide (and hence its concentration) continuously in the ocean and in sewage.
Bisulfide is sometimes confused with the disulfide
dianion, S22−, or −S–S−.
anionic ligand
that forms complexes with most metal ions. Examples include [Au(SH)2]− and (C5H5)2Ti(SH)2, derived from gold(I) chloride and titanocene dichloride
, respectively.
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
[HS]− (also commonly written [SH]− by analogy to [OH]−). This species is the conjugate base of hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
and in its turn also dissociates in sulfide
Sulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
:
- H2S → HS− + H+
- HS− → S2− + H+
In aqueous solutions, at pH lower than 7, hydrogen sulfide (H2S) is the dominant species but at pH higher than 7, bisulfide (HS−) dominates. Sulfide (S2−) is an extremely basic group and only dominates in strongly alkaline conditions at elevated pH.
A variety of salts are known, including sodium hydrosulfide
Sodium hydrosulfide
Sodium hydrosulfide is the chemical compound with the formula NaHS. This compound is the product of the half neutralization of hydrogen sulfide with sodium hydroxide. NaHS is a useful reagent for the synthesis of organic and inorganic sulfur compounds. It is a colorless solid that typically smells...
and potassium hydrosulfide
Potassium hydrosulfide
Potassium hydrosulfide is the inorganic compound with the formula KHS. This colourless salt consists of the cation K+ and the bisulfide anion [SH]−. It is the product of the half-neutralization of hydrogen sulfide with potassium hydroxide. The compound is used in the synthesis of some...
. Ammonium hydrosulfide, a component of "stink bombs" has not been isolated as a pure solid. Some compounds described as salts of the sulfide
Sulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
dianion contain primarily hydrosulfide. For example, the hydrated form of sodium sulfide
Sodium sulfide
Sodium sulfide is the name used to refer to the chemical compound Na2S, but more commonly it refers to the hydrate Na2S·9H2O. Both are colorless water-soluble salts that give strongly alkaline solutions...
, nominally with the formula Na2S · 9 H2O, is better described as NaSH · NaOH · 8 H2O.

Ultraviolet-visible spectroscopy
Ultraviolet-visible spectroscopy or ultraviolet-visible spectrophotometry refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent ranges...
. Research groups have used field spectrometers to measure the absorption due to bisulfide (and hence its concentration) continuously in the ocean and in sewage.
Bisulfide is sometimes confused with the disulfide
Disulfide
In chemistry, a disulfide usually refers to the structural unit composed of a linked pair of sulfur atoms. Disulfide usually refer to a chemical compound that contains a disulfide bond, such as diphenyl disulfide, C6H5S-SC6H5....
dianion, S22−, or −S–S−.
Coordination chemistry
SH− is a softHSAB theory
The HSAB concept is an acronym for 'hard and soft acids and bases. Also known as the Pearson acid base concept, HSAB is widely used in chemistry for explaining stability of compounds, reaction mechanisms and pathways....
anionic ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
that forms complexes with most metal ions. Examples include [Au(SH)2]− and (C5H5)2Ti(SH)2, derived from gold(I) chloride and titanocene dichloride
Titanocene dichloride
Titanocene dichloride is the organotitanium compound with the formula 2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air...
, respectively.

