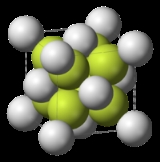
Sodium sulfide
Encyclopedia
Sodium sulfide is the name used to refer to the chemical compound
Na
2S
, but more commonly it refers to the hydrate
Na2S·9H2O
. Both are colorless water-soluble salts that give strongly alkaline
solutions. When exposed to moist air, Na2S and its hydrates emit hydrogen sulfide
, which smells much like rotten eggs or flatus.
Generally, commercially available sodium sulfide is not a unique chemical entity, but it is specified as Na2S·xH2O, where a weight percentage of Na2S is specified. Commonly available grades have around 60% Na2S by weight, which means that x is around 3. Such technical grades of sodium sulfide have a yellow appearance. These grades of sodium sulfide are marketed as 'sodium sulfide flakes'. Although the solid is yellow, solutions of it are colorless.
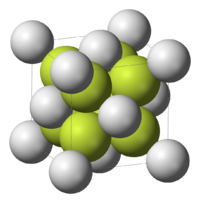 Na2S adopts the antifluorite structure, which means that the Na+ centers occupy sites of the fluoride in the CaF2 framework, and the larger S2− occupy the sites for Ca2+. In solution, the salt, by definition, dissociates. The dianion S2− does not, however, exist in appreciable amounts in water. Sulfide is too strong a base to coexist with water. Thus, the dissolution process can be described as follows:
Na2S adopts the antifluorite structure, which means that the Na+ centers occupy sites of the fluoride in the CaF2 framework, and the larger S2− occupy the sites for Ca2+. In solution, the salt, by definition, dissociates. The dianion S2− does not, however, exist in appreciable amounts in water. Sulfide is too strong a base to coexist with water. Thus, the dissolution process can be described as follows:
Sodium sulfide can oxidize when heated to sodium carbonate
and sulfur dioxide
:
with carbon, in the form of coal:
In the laboratory, the anhydrous salt can be prepared by reduction of sulfur with sodium in anhydrous ammonia. Alternatively, sulfur can be reduced by sodium in dry THF
with a catalytic amount of naphthalene
:
and paper industry in the kraft process
. It is used in water treatment as an oxygen scavenger agent, in the photographic industry to protect developer solutions from oxidation, in textile industry as a bleaching, as a desulfurising and as a dechlorinating agent and in leather trade for the sulfitisation of tanning extracts. It is used in chemical manufacturing as a sulfonation and sulfomethylation agent. It is used in the production of rubber chemicals, sulfur dyes and other chemical compounds. It is used in other applications including ore flotation, oil recovery, food preservative, making dyes, and detergent.
, which is a toxic and foul-smelling gas.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
Na
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
2S
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
, but more commonly it refers to the hydrate
Hydrate
Hydrate is a term used in inorganic chemistry and organic chemistry to indicate that a substance contains water. The chemical state of the water varies widely between hydrates, some of which were so labeled before their chemical structure was understood....
Na2S·9H2O
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
. Both are colorless water-soluble salts that give strongly alkaline
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
solutions. When exposed to moist air, Na2S and its hydrates emit hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
, which smells much like rotten eggs or flatus.
Generally, commercially available sodium sulfide is not a unique chemical entity, but it is specified as Na2S·xH2O, where a weight percentage of Na2S is specified. Commonly available grades have around 60% Na2S by weight, which means that x is around 3. Such technical grades of sodium sulfide have a yellow appearance. These grades of sodium sulfide are marketed as 'sodium sulfide flakes'. Although the solid is yellow, solutions of it are colorless.
Structure

- Na2S(s) + H2O(l) → 2Na+(aq) + HS− + OH−
Sodium sulfide can oxidize when heated to sodium carbonate
Sodium carbonate
Sodium carbonate , Na2CO3 is a sodium salt of carbonic acid. It most commonly occurs as a crystalline heptahydrate, which readily effloresces to form a white powder, the monohydrate. Sodium carbonate is domestically well-known for its everyday use as a water softener. It can be extracted from the...
and sulfur dioxide
Sulfur dioxide
Sulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
:
- 2 Na2S + 3 O2 + 2 CO2 → 2 Na2CO3 + 2 SO2
Production
Industrially Na2S is produced by reduction of Na2SO4Sodium sulfate
Sodium sulfate is the sodium salt of sulfuric acid. When anhydrous, it is a white crystalline solid of formula Na2SO4 known as the mineral thenardite; the decahydrate Na2SO4·10H2O has been known as Glauber's salt or, historically, sal mirabilis since the 17th century. Another solid is the...
with carbon, in the form of coal:
- Na2SO4 + 4 C → Na2S + 4 CO
In the laboratory, the anhydrous salt can be prepared by reduction of sulfur with sodium in anhydrous ammonia. Alternatively, sulfur can be reduced by sodium in dry THF
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
with a catalytic amount of naphthalene
Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
:
- 2 Na + S → Na2S
Uses
It is primarily used in pulpPulp (paper)
Pulp is a lignocellulosic fibrous material prepared by chemically or mechanically separating cellulose fibres from wood, fibre crops or waste paper. Wood pulp is the most common raw material in papermaking.-History:...
and paper industry in the kraft process
Kraft process
The kraft process describes a technology for conversion of wood into wood pulp consisting of almost pure cellulose fibers...
. It is used in water treatment as an oxygen scavenger agent, in the photographic industry to protect developer solutions from oxidation, in textile industry as a bleaching, as a desulfurising and as a dechlorinating agent and in leather trade for the sulfitisation of tanning extracts. It is used in chemical manufacturing as a sulfonation and sulfomethylation agent. It is used in the production of rubber chemicals, sulfur dyes and other chemical compounds. It is used in other applications including ore flotation, oil recovery, food preservative, making dyes, and detergent.
Safety
Like sodium hydroxide, sodium sulfide is strongly alkaline and can cause skin burns. Acids react with it to rapidly produce hydrogen sulfideHydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
, which is a toxic and foul-smelling gas.
External links
- chemicalland21.com Sodium sulfide.

