Bergmann degradation
Encyclopedia
The Bergmann degradation is a series of chemical reaction
s designed to remove a single amino acid
from the carboxylic acid
end of a peptide
.
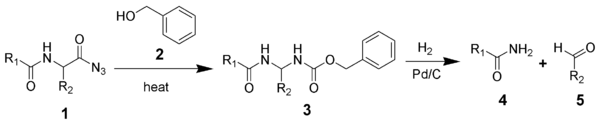 The acyl
The acyl
azide
of a peptide (1) undergoes a Curtius rearrangement
in the presence of benzyl alcohol
(2) to give a benzyl carbamate
(3). The Cbz group of intermediate 3 is removed by hydrogenolysis
to give an unsubstituted amide
(4) and an aldehyde
(5).
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s designed to remove a single amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
from the carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
end of a peptide
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
.

Acyl
An acyl group is a functional group derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids.In organic chemistry, the acyl group is usually derived from a carboxylic acid . Therefore, it has the formula RCO-, where R represents an alkyl group that is...
azide
Azide
Azide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−...
of a peptide (1) undergoes a Curtius rearrangement
Curtius rearrangement
The Curtius rearrangement , as first defined by Theodor Curtius, is a chemical reaction that involves the rearrangement of an acyl azide to an isocyanate. Several reviews have been published....
in the presence of benzyl alcohol
Benzyl alcohol
Benzyl alcohol is an organic compound with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn", thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is a useful solvent due to its polarity, low toxicity, and low vapor...
(2) to give a benzyl carbamate
Carboxybenzyl
Carboxybenzyl or Cbz or Z is an amine protecting group in organic synthesis. It is commonly used in peptide synthesis and is formed by reacting an amine with benzyl chloroformate and a weak base:It is used to protect amines from electrophiles...
(3). The Cbz group of intermediate 3 is removed by hydrogenolysis
Hydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes "lysis" by hydrogen. The heteroatom may vary, but it usually is oxygen, nitrogen, or sulfur. A related reaction is hydrogenation, where hydrogen is added to the molecule, without...
to give an unsubstituted amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
(4) and an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
(5).

