
Allopumiliotoxin
Encyclopedia
Alkaloid
Alkaloids are a group of naturally occurring chemical compounds that contain mostly basic nitrogen atoms. This group also includes some related compounds with neutral and even weakly acidic properties. Also some synthetic compounds of similar structure are attributed to alkaloids...
s. The compounds of the pumiliotoxin-A class are primarily found in the skins of frogs, toads, and other amphibians and are used as a chemical defense mechanism to ward off predators, microorganism
Microorganism
A microorganism or microbe is a microscopic organism that comprises either a single cell , cell clusters, or no cell at all...
s, and ectoparasites. The compounds were originally discovered in neotropical dendrobatid frogs, but are also found in the mantellid frogs of Madagascar
Madagascar
The Republic of Madagascar is an island country located in the Indian Ocean off the southeastern coast of Africa...
, myobatrachid frogs of Australia
Australia
Australia , officially the Commonwealth of Australia, is a country in the Southern Hemisphere comprising the mainland of the Australian continent, the island of Tasmania, and numerous smaller islands in the Indian and Pacific Oceans. It is the world's sixth-largest country by total area...
, and bufonid toad of South America
South America
South America is a continent situated in the Western Hemisphere, mostly in the Southern Hemisphere, with a relatively small portion in the Northern Hemisphere. The continent is also considered a subcontinent of the Americas. It is bordered on the west by the Pacific Ocean and on the north and east...
. Frogs possessing this defense mechanism have aposematic coloring.
Biological Activity
The Dendrobatidae family of poison-dart frogs has yielded many different alkaloids categorized into several different classes, almost all of which have shown high pharmacological activity on muscle and nerve cells.The pumiliotoxin-A class, specifically, contains many molecules which have had a favorable effect on the heart. Allopumiliotoxins, the most complex member of this class, have a wide range of biological activities, the full understanding of which has not been fully discerned due their incredible complexity and subsequent synthetic difficulties. Among allopumiliotoxins, those with a β-oriented C-7 hydroxyl group have shown greater activity in comparison to α-epimers of this position. Allopumiliotoxin 339A has been shown to stimulate sodium influx and phosphoinositide breakdown in the cerebral cortical synaptoneurosomes of guinea pigs and is one of the most active allopumiliotoxins. It is more biologically active than pumiliotoxin B, which has had similar biological affects on the secondary messenger system, causing muscle rigidity and some favorable effects on the heart.
Pumiliotoxin
Pumiliotoxin
Pumiliotoxins , are one of several toxins found in the skin of poison dart frogs. Closely related, though more toxic, are allopumiliotoxins, . Other toxins found in the skin of poison frogs include decahydroquinolines , izidines, coccinellines, and spiropyrrolizidines. Pumiliotoxins are very...
s and allopumiliotoxins are very toxic in general. Pumiliotoxin B has caused death in mice
MICE
-Fiction:*Mice , alien species in The Hitchhiker's Guide to the Galaxy*The Mice -Acronyms:* "Meetings, Incentives, Conferencing, Exhibitions", facilities terminology for events...
when 20 μg was given in injections below the skin
Nomenclature
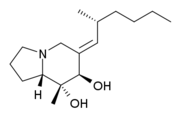
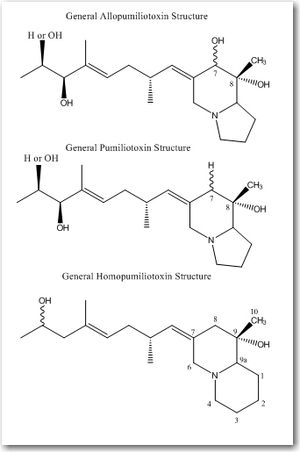
There are three divisions within the pumiliotoxin-A class: allopumiliotoxins, pumiliotoxins, and homopumiliotoxins. Once the compound’s specific class is determined, it’s given a number based on its molecular weight. These biologically active compounds are complex and have structural variations that allow for specific molecular recognition. Therefore, the way 2 isomers are differentiated is by a letter after the number. Therefore, for example, allopumiliotoxin 339A is an allopumiliotoxin with a molecular weight of 339 g/mol but there are other isomers with the same molecular weight. Allopumiliotoxin 339A has an axially oriented hydroxyl group at the 7-position in the indolizidine nucleus that differentiates it from allopumiliotoxin 339B. A (+) or (-) sign preceding the name of an allopumiliotoxin refers to the compound’s optical activity. Compounds that rotate a plane of polarized light clockwise are referred to as dextrorotatory and are preceded by a (+) sign. Compounds that rotate a plane of polarized light counterclockwise are referred to as levorotatory and are preceded by a (-) sign.Structure
The different divisions of compounds in the pumiliotoxin-A class arise from differences in the carbon backbone and/or the substituents attached to it. The difference between allopumiliotoxins and pumiliotoxinPumiliotoxin
Pumiliotoxins , are one of several toxins found in the skin of poison dart frogs. Closely related, though more toxic, are allopumiliotoxins, . Other toxins found in the skin of poison frogs include decahydroquinolines , izidines, coccinellines, and spiropyrrolizidines. Pumiliotoxins are very...
s occurs at the 7 position. At this position, allopumiliotoxins have a hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
substituent whereas pumiliotoxin
Pumiliotoxin
Pumiliotoxins , are one of several toxins found in the skin of poison dart frogs. Closely related, though more toxic, are allopumiliotoxins, . Other toxins found in the skin of poison frogs include decahydroquinolines , izidines, coccinellines, and spiropyrrolizidines. Pumiliotoxins are very...
s have a hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
. Both have methyl and hydroxyl groups at the C-8 position. Homopumiliotoxins contain a quinolizidine
Quinolizidine
Quinolizidine is a nitrogen-containing heterocyclic compound. Some alkaloids are derivatives of quinolizidine....
ring in the place of the indolizidine ring and methyl and hydroxyl groups at its C-9 position. All three contain an alkylidenyl side-chain.
Isolation
As stated previously, these alkaloids were first discovered in the skins of frogs. They can be isolated from the frog’s skin by mincing the skin and extracting the compounds by triturationTrituration
Trituration is the name of several different methods of processing materials. Trituration is also the name of the process for reducing the particle size of a substance by grinding, as by grinding of powders in a mortar with a pestle. Trituration additionally refers to the production of a...
. A series of extractions involving an acid-base extraction are needed to isolate the allopumiliotoxins. The skins of the frogs may contain a number of different allopumiliotoxins. For example, the skin of Dendrobates
Dendrobates
Dendrobates is a genus of poison dart frogs native to South America. It once contained all poison dart frogs; until recently, frogs such as Dendrobates pumilio and Dendrobates terribilis were scientifically valid names...
tricolor was found to contain alkalkoids 251D, 271, 341A, and 323B. Also, different frogs contain different alkaloids in their skin. Dendrobates auratus, for example, was found to contain (+)-allopumiliotoxin 339A (a compound not present in the skin of Dendrobates tricolor).
Synthesis
Allopumiliotoxins are very biologically useful but are rare in nature. For this reason, many groups have researched syntheses for various alkaloids of this type. The main problem with allopumiliotoxin synthesis arises from the alkylidene side chain because the stereochemistryStereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
of it can be difficult to control by Wittig
Wittig
Wittig is a surname, and may refer to:* Curt Wittig , American chemist* David Wittig , American executive* Edward Wittig , Polish sculptor* Georg Wittig , German chemist...
-type functionalizations.
Total synthesis of (+)-allopumiliotoxin 267A was achieved using a chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
dihydropyridone intermediate that was formed from the addition of ethyl lithiopropiolate to the N-acylpyridinium salt which results from the reaction of (+)-trans-2-(α-cumyl)cyclohexyl chloroformate and 4-methoxy-3-methyl-5(triisopropylsilyl)pyridine. This intermediate was then subjected to various additions and oxidations to yield the final allopumiliotoxin. The synthesis of (+)-allopumiliotoxin 323B’ has also been achieved using an intermediate from the previous synthesis.
(+)-Allopumiliotoxin 339A has been synthesized using an iodide-promoted iminium ion alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
cyclization followed by condensation with acetylenic salt. Subsequent reactions led to the enantiopure product after 16 steps and a 7.5% yield. Other synthetic methods have been carried out for this molecule. One of which was achieved through the use of a Nozaki-Kishi Cyclization. Allopumiliotoxin 267A
Allopumiliotoxin 267A
Allopumiliotoxin 267A is a toxin found in the skin of several poison frogs of the family Dendrobates. It is a member of the class of compounds known as allopumiliotoxins. The frogs produce the toxin by modifying the original version, pumiliotoxin 251D. It has been tested on mice and found to be...
was synthesized using a similar cyclization.
Analysis
The pumiliotoxin-A class compounds are typically analyzed by GC-MS because the different classes show different prominent peaks. Allopumiliotoxins show corresponding ions of C4H8N+(m/z 70) and C10H16NO2+(m/z 182). The mass spectra of pumiliotoxinPumiliotoxin
Pumiliotoxins , are one of several toxins found in the skin of poison dart frogs. Closely related, though more toxic, are allopumiliotoxins, . Other toxins found in the skin of poison frogs include decahydroquinolines , izidines, coccinellines, and spiropyrrolizidines. Pumiliotoxins are very...
s show prominent ions of C4H8N+ (m/z 70) and C10H16NO+ (m/z 166). Homopumiliotoxins exhibit prominent mass spectral fragment ions of C5H10N+ (m/z 84) and C11H18NO+ (m/z 180).

