
Unbihexium
Encyclopedia
Unbihexium also known as eka
-plutonium
or element 126, is a hypothetical chemical element
with atomic number
126 and symbol Ubh. It is of interest because of its location at the peak of the hypothesized island of stability
.
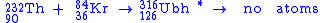
A high energy alpha particle
was observed and taken as possible evidence for the synthesis of unbihexium. Recent research suggests that this is highly unlikely as the sensitivity of experiments performed in 1971 would have been several orders of magnitude too low according to current understanding.
To date, no other attempt has been made to synthesize unbihexium.
s of unbihexium that would be considerably longer-lived than others are 310Ubh and 322Ubh.
Another way to synthesize unbihexium would be to overshoot it by fusion of 130Te and 204Hg; successive alpha decay of the compound nucleus 334Utb would land right on 322Ubh (predicted to be relatively stable). 130Te constitutes about 34% of the natural element; however, 204Hg only constitutes about 7% of natural mercury.
g-electron atoms, although the g-block's position left of the f-block
is speculative. The expected electron configuration is [Uuo]5g6 8s2 although there may be a smearing out of the energies of 5g, 6f and 7d orbitals.
Recent calculations have suggested a stable monofluoride
UbhF may exist, resulting from a bonding interaction between the 5g orbital
on Ubh and the 2p orbital on fluorine
. Other predicted oxidation states include III, IV, VI, and VIII.
Mendeleev's predicted elements
Professor Dmitri Mendeleev published the first Periodic Table of the Atomic Elements in 1869 based on properties which appeared with some regularity as he laid out the elements from lightest to heaviest....
-plutonium
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
or element 126, is a hypothetical chemical element
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
with atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
126 and symbol Ubh. It is of interest because of its location at the peak of the hypothesized island of stability
Island of stability
The island of stability in nuclear physics describes a set of as-yet undiscovered isotopes of transuranium elements which are theorized to be much more stable than others...
.
History
The first attempt to synthesize unbihexium was performed in 1971 by Bimbot et al. using the hot fusion reaction: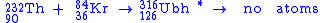
A high energy alpha particle
Alpha particle
Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is classically produced in the process of alpha decay, but may be produced also in other ways and given the same name...
was observed and taken as possible evidence for the synthesis of unbihexium. Recent research suggests that this is highly unlikely as the sensitivity of experiments performed in 1971 would have been several orders of magnitude too low according to current understanding.
To date, no other attempt has been made to synthesize unbihexium.
Target-projectile combinations leading to Z=126 compound nuclei
The table below contains various combinations of targets and projectiles (both at max no. of neutrons) which could be used to form compound nuclei with Z=126. The only practical isotopeIsotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s of unbihexium that would be considerably longer-lived than others are 310Ubh and 322Ubh.
| Target | Projectile | CN | Attempt result |
|---|---|---|---|
| 182Hf | 136Xe | 318Ubh | |
| 232Th | 84Kr | 316Ubh | |
| 243Cm | 67Zn | 310Ubh | |
| 248Cf | 62Ni | 310Ubh | |
| 249Cf | 61Ni | 310Ubh | |
| 251Es | 59Co | 310Ubh | |
| 254Fm | 56Fe | 310Ubh | |
| 257Md | 53Mn | 310Ubh | |
| 260Rf | 50Ti | 310Ubh | |
| 271Sg | 48Ca | 319Ubh |
Another way to synthesize unbihexium would be to overshoot it by fusion of 130Te and 204Hg; successive alpha decay of the compound nucleus 334Utb would land right on 322Ubh (predicted to be relatively stable). 130Te constitutes about 34% of the natural element; however, 204Hg only constitutes about 7% of natural mercury.
Stable unbihexium
Calculations according to the Hartree-Fock-Bogoliubov Method using the non-relativistic Skyrme interaction have proposed Z=126 as a closed proton shell. In this region of the periodic table, N=184 and N=196 have been suggested as closed neutron shells. Therefore the isotopes of most interest are 310Ubh and 322Ubh, for these might be considerably longer-lived than other isotopes.Predicted chemistry
Unbihexium is predicted to belong to a new block of valenceValence electron
In chemistry, valence electrons are the electrons of an atom that can participate in the formation of chemical bonds with other atoms. Valence electrons are the "own" electrons, present in the free neutral atom, that combine with valence electrons of other atoms to form chemical bonds. In a single...
g-electron atoms, although the g-block's position left of the f-block
F-block
The f-block of the periodic table of the elements consists of those elements whose atoms or ions have valence electrons in f-orbitals. Actual electronic configurations may be slightly different from what is predicted by the Aufbau principle...
is speculative. The expected electron configuration is [Uuo]5g6 8s2 although there may be a smearing out of the energies of 5g, 6f and 7d orbitals.
Recent calculations have suggested a stable monofluoride
Fluoride
Fluoride is the anion F−, the reduced form of fluorine when as an ion and when bonded to another element. Both organofluorine compounds and inorganic fluorine containing compounds are called fluorides. Fluoride, like other halides, is a monovalent ion . Its compounds often have properties that are...
UbhF may exist, resulting from a bonding interaction between the 5g orbital
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
on Ubh and the 2p orbital on fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
. Other predicted oxidation states include III, IV, VI, and VIII.
See also
- Island of stabilityIsland of stabilityThe island of stability in nuclear physics describes a set of as-yet undiscovered isotopes of transuranium elements which are theorized to be much more stable than others...
: UnunquadiumUnunquadiumUnunquadium is the temporary name of a radioactive chemical element with the temporary symbol Uuq and atomic number 114. There is no proposed name yet, although flerovium has been discussed in the media.About 80 decays of atoms of...
–UnbiniliumUnbiniliumUnbinilium , also called eka-radium or element 120, is the temporary, systematic element name of a hypothetical chemical element in the periodic table that has the temporary symbol Ubn and has the atomic number 120....
–Unbihexium - Unbipentium–Unbiseptium
- Period 8 elementPeriod 8 elementA period 8 element is any one of 50 hypothetical chemical elements belonging to an eighth period of the periodic table of the elements. They may be referred to using IUPAC systematic element names. None of these elements have been created, and it is possible that none have isotopes with stable...

