
Titration curve
Encyclopedia
Titration
Titration, also known as titrimetry, is a common laboratory method of quantitative chemical analysis that is used to determine the unknown concentration of an identified analyte. Because volume measurements play a key role in titration, it is also known as volumetric analysis. A reagent, called the...
s are often recorded on titration curves, whose compositions are generally identical: the independent variable
Independent variable
The terms "dependent variable" and "independent variable" are used in similar but subtly different ways in mathematics and statistics as part of the standard terminology in those subjects...
is the volume of the titrant, while the dependent variable is the pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
of the solution (which changes depending on the composition of the two solutions). The equivalence point
Equivalence point
The equivalence point, or stoichiometric point, of a chemical reaction when a titrant is added and is stoichiometrically equal to the amount of moles of substance present in the sample: the smallest amount of titrant that is sufficient to fully neutralize or react with the analyte...
is a significant point on the graph (the point at which all of the starting solution, usually an acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
, has been neutralized by the titrant, usually a base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
).
It can be calculated precisely by finding the second derivative
Second derivative
In calculus, the second derivative of a function ƒ is the derivative of the derivative of ƒ. Roughly speaking, the second derivative measures how the rate of change of a quantity is itself changing; for example, the second derivative of the position of a vehicle with respect to time is...
of the titration curve and computing the points of inflection (where the graph changes concavity
Concave function
In mathematics, a concave function is the negative of a convex function. A concave function is also synonymously called concave downwards, concave down, convex upwards, convex cap or upper convex.-Definition:...
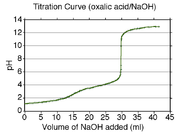
); however, in most cases, simple visual inspection of the curve will suffice (in the curve given to the right, both equivalence points are visible, after roughly 15 and 30 mL of NaOH solution has been titrated into the oxalic acid
Oxalic acid
Oxalic acid is an organic compound with the formula H2C2O4. This colourless solid is a dicarboxylic acid. In terms of acid strength, it is about 3,000 times stronger than acetic acid. Oxalic acid is a reducing agent and its conjugate base, known as oxalate , is a chelating agent for metal cations...
solution. To calculate the acid dissociation constant
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
(pKa), one must find the volume at the half-equivalence point, that is where half the amount of titrant has been added to form the next compound (here, sodium hydrogen oxalate, then disodium oxalate
Disodium oxalate
Disodium oxalate, often called simply sodium oxalate, is a sodium salt of oxalic acid with the molecular formula Na2C2O4. It is usually a white, crystalline, odorless powder, that decomposes at 250–270 °C....
). Halfway between each equivalence point, at 7.5 mL and 22.5 mL, the pH observed was about 1.5 and 4, giving the pKa.
In monoprotic acids, the point halfway between the beginning of the curve (before any titrant has been added) and the equivalence point is significant: at that point, the concentrations of the two species (the acid and conjugate base) are equal. Therefore, the Henderson-Hasselbalch equation
Henderson-Hasselbalch equation
In chemistry, the Henderson–Hasselbalch equation describes the derivation of pH as a measure of acidity in biological and chemical systems...
can be solved in this manner:
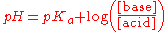
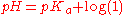
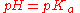
Therefore, one can easily find the pKa of the monoprotic acid by finding the pH of the point halfway between the beginning of the curve and the equivalence point, and solving the simplified equation. In the case of the sample curve, the Ka would be approximately 1.78×10-5 from visual inspection (the actual Ka2 is 1.7×10-5)
For polyprotic acids, calculating the acid dissociation constants is only marginally more difficult: the first acid dissociation constant can be calculated the same way as it would be calculated in a monoprotic acid. The second acid dissociation constant, however, is the point halfway between the first equivalence point and the second equivalence point (and so on for acids that release more than two protons, such as phosphoric acid
Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
).

