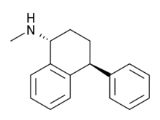
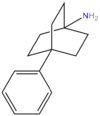
Tametraline
Encyclopedia
Tametraline is the parent of a series of chemical compounds investigated at Pfizer
that eventually led to the development of sertraline
(CP-51,974-1).
Sertraline has been called "3,4-dichloro tametraline". In the case of tametraline the cis diastereomers are totally impotent and are separated from the product because they are an unneeded contaminant (depressing the crystals melting point, etc).
1R-Methylamino-4S-phenyl-tetralin
is a potent inhibitor of NE uptake in rat brain synaptosomes, reverses reserpine
induced hypothermia in mice, and blocks uptake of [3H] into rat heart.
Tametraline is a catecholamine reuptake inhibitor that possesses certain properties that are mandatory for a psychostimulant. The benzhydryl moiety is an eminent feature and will remind viewers of related molecules such as desoxypipradrol
. Indatraline
is an indanamine analog of tetralin-based tametraline.
Two routes have been previously described, one for aryl
moieties containing electron withdrawing groups, and one for electron donating groups:
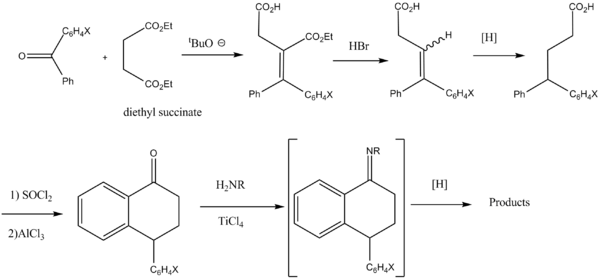
"As expected, Friedel-Crafts cyclization of the diarylbutyric acid derivatives # to the most reactive ring was observed with little or none of the alternative isomer being detected.
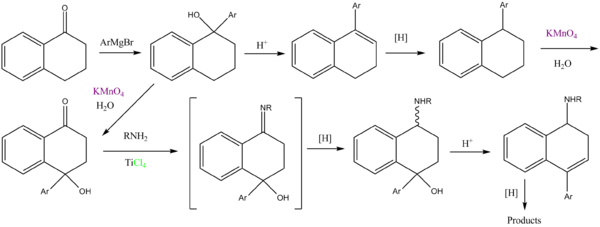
"The KMnO4 oxidation of the 1-aryl-tetralins # was observed to give 4-hydroxy-4-aryltetralones # instead of the expected tetralone # previously reported. As a result of this finding, direct oxidation of Grignard reaction
product # was attempted and found to be a more efficient route."
and acrylonitrile
is described."
http://pubs.acs.org/doi/suppl/10.1021/ol0257934/suppl_file/ol0257934_s1.pdf
http://www.organic-chemistry.org/abstracts/literature/609.shtm
Enantiopurified 4-aryl-aminotetralins IC50 (μM) >
Stereo
X
Y
NE
DA
5-HT
RS
H
H
0.018
0.15
0.84
SR
H
H
0.37
1.40
14.00
RS
Cl
H
0.019
0.052
0.084
SR
Cl
H
0.46
1.40
3.50
RS
Cl
Cl
0.01
0.044
0.039
SR
Cl
Cl
0.044
0.27
0.47
SS
Cl
Cl
1.20
1.30
0.06
RR
Cl
Cl
0.30
0.32
0.46
Interestingly, (±)-sertraline is not entirely SERT selective until it has been resolved into the SS enantiomer.
C.f. "book" values:
In terms of the trans isomers there is relatively marked separation in the activity between the RS and SR enantiomers. This stands in contrast to what has been observed in the homologous indamine class where both of the trans enantiomers possessed significant TRI activity at all three of the MA transporters.
Racemic cis 4-aryl-aminotetralins IC50 (μM) >
R1
R2
X
Y
5-HT
DA
NE
H
Me
H
H
3.50
5.10
1.86
H
Me
F
H
1.70
4.70
2.30
H
Me
Cl
H
0.26
1.38
1.41
H
Me
Br
H
0.19
1.60
1.40
H
Me
CF3
H
0.82
7.80
9.80
H
Me
H
CF3
0.25
2.54
2.55
H
Me
OMe
H
0.70
4.20
3.00
H
Me
Cl
Cl
0.07
0.52
0.72
Me
Me
H
H
1.6
10.0
0.31
Me
Me
Cl
H
0.24
5.60
1.16
Me
Me
Cl
Cl
0.07
2.00
0.40
H
H
Cl
Cl
0.40
1.25
0.25
Figures in brackets are for the N-dimethyl congeners. The primary amines are claimed to completely lack any affinity for the transporters.
c.f. "This property can be perceived as a potential advantage in that enhanced synaptosomal DA levels may be equated with undesirable stimulant properties of certain compounds in the trans series."
Sepracor has tried to patent the trans dichloro analog
During his 40 years at Pfizer, Koe authored more than 100 articles and papers.
...
Koe learned to review previous studies and to build on findings that had failed to lead to successful products. In his early work with serotonin, for example, he studied the chemical tametraline, which proved ineffective as an anti-depressant.
Tests showed the chemical functioned more as a stimulant, a use Pfizer was not interested in pursuing. Although his research had failed to yield the desired result, Koe was convinced that the development of a viable anti-depressant was within reach.
Pfizer
Pfizer, Inc. is an American multinational pharmaceutical corporation. The company is based in New York City, New York with its research headquarters in Groton, Connecticut, United States...
that eventually led to the development of sertraline
Sertraline
Sertraline hydrochloride is an antidepressant of the selective serotonin reuptake inhibitor class. It was introduced to the market by Pfizer in 1991. Sertraline is primarily used to treat major depression in adult outpatients as well as obsessive–compulsive, panic, and social anxiety disorders in...
(CP-51,974-1).
Sertraline has been called "3,4-dichloro tametraline". In the case of tametraline the cis diastereomers are totally impotent and are separated from the product because they are an unneeded contaminant (depressing the crystals melting point, etc).
1R-Methylamino-4S-phenyl-tetralin
Tetralin
Tetralin is a hydrocarbon having the chemical formula C10H12. This molecule is similar to the naphthalene chemical structure except that one ring is saturated.The compound can be synthesized in a Bergman cyclization...
is a potent inhibitor of NE uptake in rat brain synaptosomes, reverses reserpine
Reserpine
Reserpine is an indole alkaloid antipsychotic and antihypertensive drug that has been used for the control of high blood pressure and for the relief of psychotic symptoms, although because of the development of better drugs for these purposes and because of its numerous side-effects, it is rarely...
induced hypothermia in mice, and blocks uptake of [3H] into rat heart.
Tametraline is a catecholamine reuptake inhibitor that possesses certain properties that are mandatory for a psychostimulant. The benzhydryl moiety is an eminent feature and will remind viewers of related molecules such as desoxypipradrol
Desoxypipradrol
Desoxypipradrol, also known as 2-diphenylmethylpiperidine , acts as a norepinephrine-dopamine reuptake inhibitor developed by Ciba in the 1950s...
. Indatraline
Indatraline
Indatraline is a non-selective monoamine transporter inhibitor that has been shown to block the reuptake of dopamine, norepinephrine, and serotonin with effects similar to those of cocaine...
is an indanamine analog of tetralin-based tametraline.
Chemistry
See also: (and refs therein: )Two routes have been previously described, one for aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
moieties containing electron withdrawing groups, and one for electron donating groups:

"As expected, Friedel-Crafts cyclization of the diarylbutyric acid derivatives # to the most reactive ring was observed with little or none of the alternative isomer being detected.

"The KMnO4 oxidation of the 1-aryl-tetralins # was observed to give 4-hydroxy-4-aryltetralones # instead of the expected tetralone # previously reported. As a result of this finding, direct oxidation of Grignard reaction
Grignard reaction
The Grignard reaction is an organometallic chemical reaction in which alkyl- or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds...
product # was attempted and found to be a more efficient route."
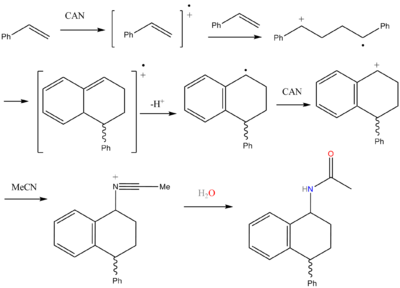
CAN radical induced dimerization of styrene
"A facile one-pot synthesis of 1-amino-4-aryl-tetralin derivatives by the CAN-induced (see also: CAN) cyclodimerization of various styrenes in acetonitrileAcetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
and acrylonitrile
Acrylonitrile
Acrylonitrile is the chemical compound with the formula C3H3N. This pungent-smelling colorless liquid often appears yellow due to impurities. It is an important monomer for the manufacture of useful plastics. In terms of its molecular structure, it consists of a vinyl group linked to a nitrile...
is described."
http://pubs.acs.org/doi/suppl/10.1021/ol0257934/suppl_file/ol0257934_s1.pdf
http://www.organic-chemistry.org/abstracts/literature/609.shtm

SAR
Certain aromatic substitutients have a potentiating effect (e.g. p-Br), whereas others negate the compounds intrinsic activity.Enantiopurified Trans and Cis Aminotetraline Derivatives
Interestingly, (±)-sertraline is not entirely SERT selective until it has been resolved into the SS enantiomer.
C.f. "book" values:
In terms of the trans isomers there is relatively marked separation in the activity between the RS and SR enantiomers. This stands in contrast to what has been observed in the homologous indamine class where both of the trans enantiomers possessed significant TRI activity at all three of the MA transporters.
Racemic Cis and Trans Aminotetraline Derivatives
|
| Racemic trans 4-aryl-aminotetralins IC50 (μM) | >|||||||
| R1 | R2 | X | Y | NE | DA | 5-HT | ||
| H | Me | H | H | 0.04 | 0.21 | 1.48 | ||
| H | Me | F | H | 0.03 | 0.22 | 0.58 | ||
| H | Me | Cl | H | 0.03 | 0.10 | 0.12 | ||
| H | Me | Br | H | 0.03 | 0.08 | 0.09 | ||
| H | Me | CF3 | H | 0.69 | 4.4 | 0.43 | ||
| H | Me | H | CF3 | 0.26 | 2.60 | 0.39 | ||
| H | Me | OMe | H | 0.15 | 0.40 | 0.38 | ||
| H | Me | Cl | Cl | 0.02 | 0.06 | 0.05 | ||
| Me | Me | H | H | 0.14 | 0.84 | 0.46 | ||
| Me | Me | Cl | H | 0.13 | 0.38 | 0.12 | ||
| Me | Me | Cl | Cl | 0.04 | 0.17 | 0.04 | ||
Figures in brackets are for the N-dimethyl congeners. The primary amines are claimed to completely lack any affinity for the transporters.
c.f. "This property can be perceived as a potential advantage in that enhanced synaptosomal DA levels may be equated with undesirable stimulant properties of certain compounds in the trans series."
See also

- EXP-561EXP-561EXP-561 is a drug which acts as a inhibitor of the reuptake of serotonin, dopamine, and norepinephrine. It was developed in the 1960s by Du Pont and was suggested as a potential antidepressant but was never marketed.-Synthesis:...
(1-amino-4-phenylbicyclo[2.2.2]octane) - cyproheptadineCyproheptadineCyproheptadine , sold under the brand name Periactin, is a first-generation antihistamine with additional anticholinergic, antiserotonergic, and local anesthetic properties.- Indications :...
[4-(5H-dibenz-[a,d]cyclohepten-5-ylidine)-1-methylpiperidine] - NefopamNefopamNefopam is a centrally-acting but non-opioid analgesic drug of the benzoxazocine chemical class which was developed by Riker Laboratories in the 1960s. It is widely used, mainly in European countries, for the relief of moderate to severe pain as an alternative to opioid analgesic drugs...
- CP-24,441 (1R, 4S-N-methyl- 4-phenyl-1,2,3,4-tetrahydro-1-naphthylamine)
- CP-39,332 (N-methyl-4-phenyl-1,2,3,4-tetrahydro-2- naphthylamine)
- JNJ-7925476JNJ-7925476JNJ-7925476 is a TRI antidepressant currently under development by Johnson & Johnson.These molecules were first prepared by Bruce E. Maryanoff, et al. during the late 1970s – 1980's...
Sepracor has tried to patent the trans dichloro analog
External links
- http://www.healyprozac.com/Book/Introduction.doc
- http://www.zoominfo.com/people/Koe_Ken_594418650.aspx
During his 40 years at Pfizer, Koe authored more than 100 articles and papers.
...
Koe learned to review previous studies and to build on findings that had failed to lead to successful products. In his early work with serotonin, for example, he studied the chemical tametraline, which proved ineffective as an anti-depressant.
Tests showed the chemical functioned more as a stimulant, a use Pfizer was not interested in pursuing. Although his research had failed to yield the desired result, Koe was convinced that the development of a viable anti-depressant was within reach.

