
Taft equation
Encyclopedia
Free-energy relationship
In physical organic chemistry, a free-energy relationship or linear Gibbs energy relation relates the logarithm of a reaction rate constant or equilibrium constant for one series of reactions with the logarithm of the rate or equilibrium constant for a related series of reactions...
(LFER) used in physical organic chemistry
Physical organic chemistry
Physical organic chemistry is the study of the interrelationships between structure and reactivity in organic molecules. It can be seen as the study of organic chemistry using tools of physical chemistry such as chemical equilibrium, chemical kinetics, thermochemistry, and quantum chemistry...
in the study of reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
s and in the development of quantitative structure activity relationships
Quantitative structure-activity relationship
Quantitative structure–activity relationship or QSPR is the process by which chemical structure is quantitatively correlated with a well defined process, such as biological activity or chemical reactivity.For example, biological activity can be expressed quantitatively as the concentration of a...
for organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s. It was developed by Robert W. Taft in 1952 as a modification to the Hammett equation
Hammett equation
The Hammett equation in organic chemistry describes a linear free-energy relationship relating reaction rates and equilibrium constants for many reactions involving benzoic acid derivatives with meta- and para-substituents to each other with just two parameters: a substituent constant and a...
. While the Hammett equation accounts for how field, inductive
Inductive effect
In chemistry and physics, the inductive effect is an experimentally observable effect of the transmission of charge through a chain of atoms in a molecule by electrostatic induction...
, and resonance
Mesomeric effect
The mesomeric effect or resonance effect in chemistry is a property of substituents or functional groups in a chemical compound. The effect is used in a qualitative way and describes the electron withdrawing or releasing properties of substituents based on relevant resonance structures and is...
effects influence reaction rates, the Taft equation also describes the steric effects
Steric effects
Steric effects arise from the fact that each atom within a molecule occupies a certain amount of space. If atoms are brought too close together, there is an associated cost in energy due to overlapping electron clouds , and this may affect the molecule's preferred shape and reactivity.-Steric...
of a substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
. The Taft equation is written as:
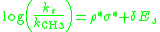
where log(ks/kCH3) is the ratio of the rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
of the substituted reaction compared to the reference reaction, σ* is the polar substituent constant that describes the field and inductive effects of the substituent, Es is the steric substituent constant, ρ* is the sensitivity factor for the reaction to polar effect
Polar effect
The Polar effect or electronic effect in chemistry is the effect exerted by a substituent on modifying electrostatic forces operating on a nearby reaction center...
s, and δ is the sensitivity factor for the reaction to steric effects.
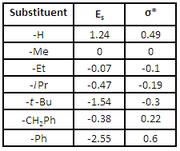
Polar Substituent Constants, σ*
Polar substituent constants describe the way a substituent will influence a reaction through polar (inductive, field, and resonance) effects. To determine σ* Taft studied the hydrolysisHydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of methyl esters (RCOOMe). The use of ester hydrolysis rates to study polar effects was first suggested by Ingold in 1930. The hydrolysis of esters can occur through either acid and base catalyzed mechanisms
Acid catalysis
In acid catalysis and base catalysis a chemical reaction is catalyzed by an acid or a base. The acid is often the proton and the base is often a hydroxyl ion. Typical reactions catalyzed by proton transfer are esterfications and aldol reactions. In these reactions the conjugate acid of the carbonyl...
, both of which proceed through a tetrahedral
Tetrahedral molecular geometry
In a tetrahedral molecular geometry a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1 ≈ 109.5° when all four substituents are the same, as in CH4. This molecular geometry is common throughout the first...
intermediate
Reactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
. In the base catalyzed mechanism the reactant goes from a neutral species to negatively charged intermediate in the rate determining (slow) step
Rate-determining step
The rate-determining step is a chemistry term for the slowest step in a chemical reaction. The rate-determining step is often compared to the neck of a funnel; the rate at which water flows through the funnel is determined by the width of the neck, not by the speed at which water is poured in. In...
, while in the acid catalyzed mechanism a positively charged reactant goes to a positively charged intermediate.
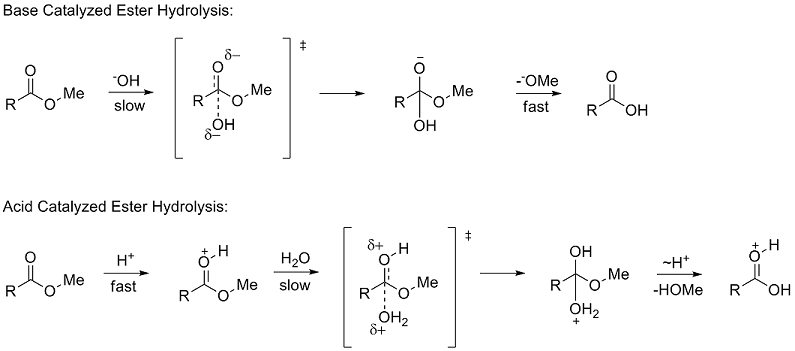
Due to the similar tetrahedral intermediates, Taft proposed that under identical conditions any steric factors should be nearly the same for the two mechanisms and therefore would not influence the ratio of the rates. However, because of the difference in charge buildup in the rate determining steps it was proposed that polar effects would only influence the reaction rate of the base catalyzed reaction since a new charge was formed. He defined the polar substituent constant σ* as:
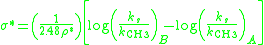
where log(ks/kCH3)B is the ratio of the rate of the base catalyzed reaction compared to the reference reaction, log(ks/kCH3)A is ratio of a rate of the acid catalyzed reaction compared to the reference reaction, and ρ* is a reaction constant that describes the sensitivity of the reaction series. For the definition reaction series, ρ* was set to 1 and R = methyl was defined as the reference reaction (σ* = zero). The factor of 1/2.48 is included to make σ* similar in magnitude to the Hammett σ values.
Steric Substituent Constants, Es
Although the acid catalyzed and base catalyzed hydrolysis of esters gives transition stateTransition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
s for the rate determining steps that have differing charge densities
Charge density
The linear, surface, or volume charge density is the amount of electric charge in a line, surface, or volume, respectively. It is measured in coulombs per meter , square meter , or cubic meter , respectively, and represented by the lowercase Greek letter Rho . Since there are positive as well as...
, their structures differ only by two hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
atoms. Taft thus assumed that steric effects would influence both reaction mechanisms equally. Due to this, the steric substituent constant Es was determined from solely the acid catalyzed reaction, as this would not include polar effects. Es was defined as:

where ks is the rate of the studied reaction and kCH3 is the rate of the reference reaction (R = methyl). δ is a reaction constant that describes the susceptibility of a reaction series to steric effects. For the definition reaction series δ was set to 1 and Es for the reference reaction was set to zero. This equation is combined with the equation for σ* to give the full Taft equation.
From comparing the Es values for methyl, ethyl
Ethyl group
In chemistry, an ethyl group is an alkyl substituent derived from ethane . It has the formula -C2H5 and is very often abbreviated -Et.Ethylation is the formation of a compound by introduction of the ethyl functional group, C2H5....
, isopropyl
Isopropyl
In organic chemistry, isopropyl is a propyl with a group attached to the secondary carbon. If viewed as a functional group an isopropyl is an organic compound with a propyl group attached at its secondary carbon.The bond is therefore on the middle carbon....
, and tert-butyl, it is seen that the value increases with increasing steric bulk. However, because context will have an effect on steric interactions some Es values can be larger or smaller than expected. For example, the value for phenyl
Phenyl group
In organic chemistry, the phenyl group or phenyl ring is a cyclic group of atoms with the formula C6H5. Phenyl groups are closely related to benzene. Phenyl groups have six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the...
is much larger than that for tert-butyl. When comparing these groups using another measure of steric bulk, axial strain values
A value
thumb|400px|right| The A-value for a [[methyl]] group is 1.74 as derived from the [[chemical equilibrium]] above. This means it costs 1.74 [[kcal/mol]] of energy to have a methyl group in the axial position compared to the equatorial position....
, the tert-butyl group is larger.
Other Steric Parameters for LFERs
In addition to Taft’s steric parameter Es, other steric parameters that are independent of kinetic data have been defined. Charton has defined values v that are derived from van der Waals radiiVan der Waals radius
The van der Waals radius, r, of an atom is the radius of an imaginary hard sphere which can be used to model the atom for many purposes. It is named after Johannes Diderik van der Waals, winner of the 1910 Nobel Prize in Physics, as he was the first to recognise that atoms had a finite size and to...
. Using molecular mechanics
Molecular mechanics
Molecular mechanics uses Newtonian mechanics to model molecular systems. The potential energy of all systems in molecular mechanics is calculated using force fields...
, Meyers has defined Va values that are derived from the volume of the portion of the substituent that is within 0.3 nm of the reaction center.
Polar Sensitivity Factor, ρ*
Similar to ρ values for Hammett plots, the polar sensitivity factor ρ* for Taft plots will describe the susceptibility of a reaction series to polar effects. When the steric effects of substituents do not significantly influence the reaction rate the Taft equation simplifies to a form of the Hammett equation: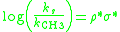
The polar sensitivity factor ρ* can be obtained by plotting the ratio of the measured reaction rates (ks) compared to the reference reaction (kCH3) versus the σ* values for the substituents. This plot will give a straight line with a slope
Slope
In mathematics, the slope or gradient of a line describes its steepness, incline, or grade. A higher slope value indicates a steeper incline....
equal to ρ*. Similar to the Hammett ρ value:
- If ρ* > 1, the reaction accumulates negative charge in the transition state and is accelerated by electron withdrawing groupsPolar effectThe Polar effect or electronic effect in chemistry is the effect exerted by a substituent on modifying electrostatic forces operating on a nearby reaction center...
. - If 1 > ρ* > 0, negative charge is built up and the reaction is mildly sensitive to polar effects.
- If ρ* = 0, the reaction is not influenced by polar effects.
- If 0 > ρ* > -1, positive charge is built up and the reaction is mildly sensitive to polar effects.
- If -1 > ρ*, the reaction accumulates positive charge and is accelerated by electron donating groupsPolar effectThe Polar effect or electronic effect in chemistry is the effect exerted by a substituent on modifying electrostatic forces operating on a nearby reaction center...
.
Steric Sensitivity Factor, δ
Similar to the polar sensitivity factor, the steric sensitivity factor δ for a new reaction series will describe to what magnitude the reaction rate is influenced by steric effects. When a reaction series is not significantly influenced by polar effects, the Taft equation reduces to: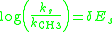
A plot of the ratio of the rates versus the Es value for the substituent will give a straight line with a slope equal to δ. Similarly to the Hammett ρ value, the magnitude of δ will reflect to what extent a reaction is influenced by steric effects:
- A very steep slope will correspond to high steric sensitivity, while a shallow slope will correspond to little to no sensitivity.
- If δ is negative, increasing steric bulk decreases the reaction rate and steric effects are greater in the transition state.
- If δ is positive, increasing steric bulk increases the reaction rate and sterics effects are lessened in the transition state.
Reactions Influenced by Polar and Steric Effects
When both steric and polar effects influence the reaction rate the Taft equation can be solved for both ρ* and δ through the use of standard least squaresLeast squares
The method of least squares is a standard approach to the approximate solution of overdetermined systems, i.e., sets of equations in which there are more equations than unknowns. "Least squares" means that the overall solution minimizes the sum of the squares of the errors made in solving every...
methods for determining a bivariant regression plane. Taft outlined the application of this method to solving the Taft equation in a 1957 paper.
Taft Plots in QSAR
The Taft equation is often employed in biological chemistryBiochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
and medicinal chemistry
Medicinal chemistry
Medicinal chemistry and pharmaceutical chemistry are disciplines at the intersection of chemistry, especially synthetic organic chemistry, and pharmacology and various other biological specialties, where it is involved with design, chemical synthesis and development for market of pharmaceutical...
for the development of quantitative structure activity relationships
Quantitative structure-activity relationship
Quantitative structure–activity relationship or QSPR is the process by which chemical structure is quantitatively correlated with a well defined process, such as biological activity or chemical reactivity.For example, biological activity can be expressed quantitatively as the concentration of a...
(QSAR). In a recent example, Sandri and co-workers have used Taft plots in studies of polar effects in the aminolysis
Aminolysis
Aminolysis is any chemical reaction in which a molecule is split into two parts by reacting with a molecule of ammonia or an amine.An example of an aminolysis reaction is the replacement of a halogen in an alkyl group by an amine and the elimination of hydrogen halide .Another common example is...
of β-lactams
Beta-lactam
A β-lactam ring, is a four-membered lactam. It is named as such, because the nitrogen atom is attached to the β-carbon relative to the carbonyl...
. They have looked at the binding of β-lactams to a poly(ethyleneimine) polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
, which functions as a simple mimic for human serum albumin
Human serum albumin
Human serum albumin is the most abundant protein in human blood plasma. It is produced in the liver. Albumin constitutes about half of the blood serum protein...
(HSA). The formation of a covalent bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
between penicillins and HSA as a result of aminolysis with lysine
Lysine
Lysine is an α-amino acid with the chemical formula HO2CCH4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG....
residues is believed to be involved in penicillin allergies. As a part of their mechanistic studies Sandri and co-workers plotted the rate of aminolysis versus calculated σ* values for 6 penicillins and found no correlation, suggesting that the rate is influenced by other effects in addition to polar and steric effects

