Simmons-Smith reaction
Encyclopedia
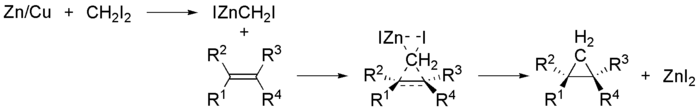
The Simmons–Smith reaction is an organic
cheletropic reaction
in which a carbenoid
reacts with an alkene
(or alkyne
) to form a cyclopropane
. It is named after Howard Ensign Simmons, Jr.
and R. D. Smith. Because the methylene
unit is delivered to both carbons of the alkene simultaneously, the configuration of the double bond is preserved in the product and the reaction is stereospecific.
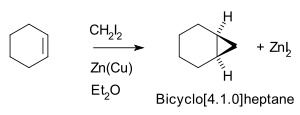
Thus, cyclohexene
, diiodomethane
, and a zinc-copper couple
(as iodomethylzinc iodide
, ICH2ZnI) yield norcarane
(bicyclo[4.1.0]heptane).
Alternatively, diethylzinc
may be used instead of the zinc-copper couple.
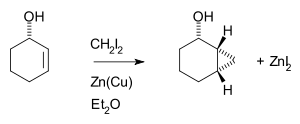
The Simmons–Smith reaction is generally subject to steric effects
, and thus cyclopropanation usually takes place on the less hindered face. However, when a hydroxy substituent is present in the substrate in proximity to the double bond, the zinc coordinates with the hydroxy substituent, directing cyclopropanation cis to the hydroxyl group (which may not correspond to cyclopropanation of the sterically most accessible face of the double bond):
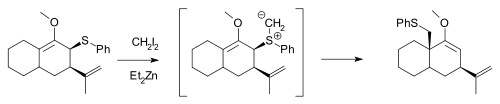
The Simmons–Smith reagent, namely diiodomethane and diethylzinc, can react with allylic thioether
s to generate sulfur
ylides, which can subsequently undergo a 2,3-sigmatropic rearrangement
, and will not cyclopropanate an alkene in the same molecule unless excess Simmons–Smith reagent is used:
compounds (see bisoxazoline ligand
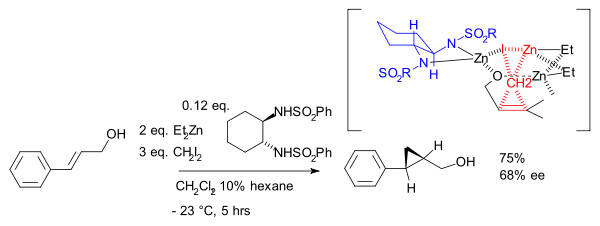
) exist since 1966, the asymmetric Simmons–Smith reaction was introduced in 1992 with a reaction of cinnamyl alcohol
with diethylzinc
, diiodomethane
and a chiral disulfonamide
in dichloromethane
:
The hydroxyl
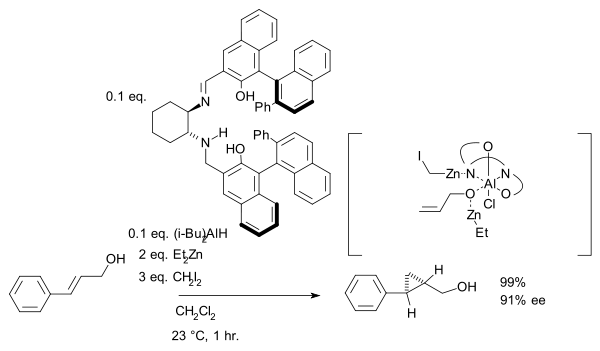
group is a prerequisite serving as an anchor for zinc. In another version of this reaction the ligand is based on salen
and Lewis acid
DIBAL is added:
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
cheletropic reaction
Cheletropic reaction
thumb|300px|right|Pericyclic ReactionsCheletropic reactions are a type of pericyclic reaction. A pericyclic reaction is one that involves a transition state with a cyclic array of atoms and an associated cyclic array of interacting orbitals. A reorganization of σ and π bonds occurs in this cyclic...
in which a carbenoid
Carbenoid
In chemistry a carbenoid is a reactive intermediate that shares reaction characteristics with a carbene. In the Simmons-Smith reaction the carbenoid intermediate is a zinc / iodine complex that takes the form of...
reacts with an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
(or alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
) to form a cyclopropane
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
. It is named after Howard Ensign Simmons, Jr.
Howard Ensign Simmons, Jr.
Howard Ensign Simmons, Jr. was an American chemist who discovered the Simmons-Smith reaction.In 1976, Dr. Simmons served as Chair of the Organic division of the American Chemical Society.-References:...
and R. D. Smith. Because the methylene
Methylene
Methylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
unit is delivered to both carbons of the alkene simultaneously, the configuration of the double bond is preserved in the product and the reaction is stereospecific.
Thus, cyclohexene
Cyclohexene
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes...
, diiodomethane
Diiodomethane
Diiodomethane or methylene iodide, commonly abbreviated "MI", is a liquid organoiodine compound. It is insoluble in water, but soluble in ether and alcohol. It has a relatively high refractive index of 1.741, and a surface tension of 0.0508 N·m−1...
, and a zinc-copper couple
Zinc-copper couple
Zinc-copper couple is an alloy of zinc and copper that is employed as a reagent in organic synthesis. The “couple” was popularized after the report by Simmons and Smith in 1959 of its application as an activated source of zinc required for formation of an organozinc reagent in the Simmons-Smith...
(as iodomethylzinc iodide
Iodomethylzinc iodide
Iodomethylzinc iodide is the active reagent in the Simmons-Smith reaction. For example, iodomethylzinc iodide, formed in situ from diiodomethane and a zinc-copper couple reacts with cyclohexene to give norcarane ....
, ICH2ZnI) yield norcarane
Norcarane
Norcarane, or bicyclo[4.1.0]heptane, is a colorless liquid. It is an organic compound prepared using the Simmons-Smith reaction, by the action of diiodomethane and a zinc-copper couple on cyclohexene in diethyl ether....
(bicyclo[4.1.0]heptane).
Alternatively, diethylzinc
Diethylzinc
Diethylzinc 2Zn, or DEZn, is a highly pyrophoric organozinc compound consisting of a zinc center bound to two ethyl groups. This colourless liquid is an important reagent in organic chemistry and available commercially as a solution in hexanes, heptane, or toluene.-Synthesis:Edward Frankland first...
may be used instead of the zinc-copper couple.
The Simmons–Smith reaction is generally subject to steric effects
Steric effects
Steric effects arise from the fact that each atom within a molecule occupies a certain amount of space. If atoms are brought too close together, there is an associated cost in energy due to overlapping electron clouds , and this may affect the molecule's preferred shape and reactivity.-Steric...
, and thus cyclopropanation usually takes place on the less hindered face. However, when a hydroxy substituent is present in the substrate in proximity to the double bond, the zinc coordinates with the hydroxy substituent, directing cyclopropanation cis to the hydroxyl group (which may not correspond to cyclopropanation of the sterically most accessible face of the double bond):
The Simmons–Smith reagent, namely diiodomethane and diethylzinc, can react with allylic thioether
Thioether
A thioether is a functional group in organosulfur chemistry with the connectivity C-S-C as shown on right. Like many other sulfur-containing compounds, volatile thioethers have foul odors. A thioether is similar to an ether except that it contains a sulfur atom in place of the oxygen...
s to generate sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
ylides, which can subsequently undergo a 2,3-sigmatropic rearrangement
2,3-sigmatropic rearrangement
2,3-Sigmatropic rearrangements are a type of sigmatropic rearrangements and can be classified into two types. Rearrangements of allylic sulfoxides, amine oxides, selenoxides are neutral. Rearrangements of carbanions of allyl ethers are anionic. The general scheme for this kind of rearrangement...
, and will not cyclopropanate an alkene in the same molecule unless excess Simmons–Smith reagent is used:
Asymmetric Simmons–Smith reaction
Although asymmetric cyclopropanation methods based on diazoDiazo
Diazo refers to a type of organic compound called diazo compound that has two linked nitrogen atoms as a terminal functional group. The general formula is R2C=N2. The simplest example of a diazo compound is diazomethane...
compounds (see bisoxazoline ligand
Bisoxazoline ligand
In chemistry, bisoxazoline ligands are chiral ligands based on a bis oxazoline skeleton and used in combination with a metal compound in asymmetric synthesis as a chiral catalyst . Three frequently encountered such ligands are PyBOX, tBuBOX and PhBOX...
) exist since 1966, the asymmetric Simmons–Smith reaction was introduced in 1992 with a reaction of cinnamyl alcohol
Cinnamyl alcohol
Cinnamyl alcohol is an organic compound that is found in esterified form in storax, balsam Peru and cinnamon leaves. It forms a white crystalline solid when pure, or a yellow oil when even slightly impure. It can be produced by the hydrolysis of storax....
with diethylzinc
Diethylzinc
Diethylzinc 2Zn, or DEZn, is a highly pyrophoric organozinc compound consisting of a zinc center bound to two ethyl groups. This colourless liquid is an important reagent in organic chemistry and available commercially as a solution in hexanes, heptane, or toluene.-Synthesis:Edward Frankland first...
, diiodomethane
Diiodomethane
Diiodomethane or methylene iodide, commonly abbreviated "MI", is a liquid organoiodine compound. It is insoluble in water, but soluble in ether and alcohol. It has a relatively high refractive index of 1.741, and a surface tension of 0.0508 N·m−1...
and a chiral disulfonamide
Sulfonamide (chemistry)
In chemistry, the sulfonamide functional group is -S2-NH2, a sulfonyl group connected to an amine group.A sulfonamide is a compound that contains this group. The general formula is RSO2NH2, where R is some organic group. For example, "methanesulfonamide" is CH3SO2NH2...
in dichloromethane
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
:
The hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group is a prerequisite serving as an anchor for zinc. In another version of this reaction the ligand is based on salen
Salen ligand
Salen is the abbreviation for a popular chelating ligand used in coordination chemistry and homogeneous catalysis. The name salen is a contraction for salicylaldehyde and ethylenediamine. The ligand is a bright yellow micaceous solid that is soluble in polar organic solvents.-Nomenclature:The...
and Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
DIBAL is added: