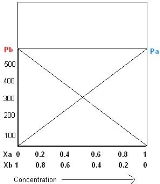
Raoult's law
Encyclopedia
Established by François-Marie Raoult
in 1882, Raoult's law states:
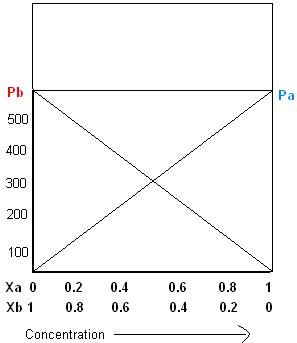
Once the components in the solution have reached equilibrium
, the total vapor pressure p of the solution is:

and the individual vapor pressure for each component is

where
Consequently, as the number of components in a solution increases, the individual vapor pressures decrease, since the mole fraction of each component decreases with each additional component. If a pure solute which has zero vapor pressure (it will not evaporate) is dissolved in a solvent, the vapor pressure of the final solution will be lower than that of the pure solvent.
This law is strictly valid only under the assumption that the chemical interactions
between the two liquids is equal to the bonding within the liquids: the conditions of an ideal solution
. Therefore, comparing actual measured vapor pressures to predicted values from Raoult's law allows information about the relative strength of bonding between liquids to be obtained. If the measured value of vapor pressure is less than the predicted value, fewer molecule
s have left the solution than expected. This is put down to the strength of bonding between the liquids being greater than the bonding within the individual liquids, so fewer molecules have enough energy to leave the solution. Conversely, if the vapor pressure is greater than the predicted value more molecules have left the solution than expected, due to the bonding between the liquids being less strong than the bonding within each.
The vapor pressure
and composition in equilibrium with a solution can yield valuable information regarding the thermodynamic properties of the liquids involved. Raoult’s law relates the vapor pressure of components to the composition of the solution. The law assumes ideal behavior. It gives a simple picture of the situation just as the ideal gas law
does. The ideal gas law is very useful as a limiting law. As the interactive forces between molecules and the volume of the molecules approaches zero, so the behavior of gases approach the behavior of the ideal gas.
Raoult’s law is similar in that it assumes that the physical properties of the components are identical. The more similar the components are, the more their behavior approaches that described by Raoult’s law. For example, if the two components differ only in isotopic
content, then the vapor pressure of each component will be equal to the vapor pressure of the pure substance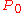 times the mole fraction in the solution. This is Raoult’s law.
times the mole fraction in the solution. This is Raoult’s law.
Using the example of a solution of two liquids, A and B, if no other gases are present, then the total vapor pressure p above the solution is equal to the weighted sum of the "pure" vapor pressures of the two components, pA and pB. Thus the total pressure above solution of A and B would be
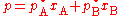
as a solution
for which the chemical potential
of component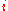 is:
is: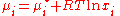 ,
,
where µ*i is the chemical potential of pure i.
If the system is at equilibrium
, then the chemical potential
of the component i must be the same in the liquid solution and in the vapor
above it. That is,
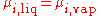
Assuming the liquid
is an ideal solution, and using the formula for the chemical potential of a gas, gives:
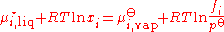
where ƒi is the fugacity
of the vapor
of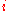 and
and  indicates reference state.
indicates reference state.
The corresponding equation for pure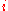 in equilibrium with its (pure) vapor is:
in equilibrium with its (pure) vapor is:
where * indicates the pure component.
Subtracting both equations gives us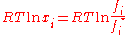
which re-arranges to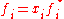
The fugacities
can be replaced by simple pressure
s if the vapor
of the solution behaves ideally
i.e.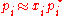
which is Raoult’s Law.
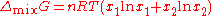
This is always negative, so mixing is spontaneous. However the expression is, apart from a factor –T, equal to the entropy of mixing. This leaves no room at all for an enthalpy effect and implies that ΔmixH must be equal to zero and this can only be if the interactions U between the molecules are indifferent.
It can be shown using the Gibbs–Duhem equation that if Raoult's law holds over the entire concentration range x = 0–1 in a binary solution then, for the second component, the same must also hold.
If the deviations from ideality are not too strong, Raoult's law will still be valid in a narrow concentration range when approaching x = 1 for the majority phase (the solvent). The solute will also show a linear limiting law but with a different coefficient. This law is known as Henry's law
.
The presence of these limited linear regimes has been experimentally verified in a great number of cases.
In a perfectly ideal system, where ideal liquid and ideal vapor are assumed, a very useful equation emerges if Raoult's law is combined with Dalton's Law
.
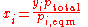
 ). The second, the activity coefficient (
). The second, the activity coefficient (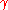 ), is a correction for interactions in the liquid phase between the different molecules.
), is a correction for interactions in the liquid phase between the different molecules.
This modified or extended Raoult's law is then written:
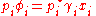
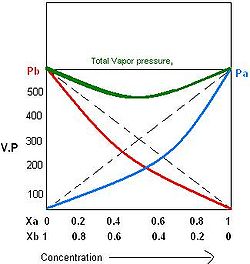 Many pairs of liquids are present in which there is no uniformity of attractive forces i.e. the adhesive and cohesive forces of attraction are not uniform between the two liquids, so that they show deviation from the Raoult's law which is applied only to ideal solutions.
Many pairs of liquids are present in which there is no uniformity of attractive forces i.e. the adhesive and cohesive forces of attraction are not uniform between the two liquids, so that they show deviation from the Raoult's law which is applied only to ideal solutions.
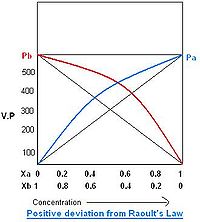 For example, chloroform and acetone show such an attraction by formation of a hydrogen bond.
For example, chloroform and acetone show such an attraction by formation of a hydrogen bond.
François-Marie Raoult
François-Marie Raoult was a French chemist who conducted research into the behavior of solutions, especially their physical properties.- Life and work :Raoult was born at Fournes, in the département of Nord...
in 1882, Raoult's law states:
- the vapor pressureVapor pressureVapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
of an ideal solutionIdeal solutionIn chemistry, an ideal solution or ideal mixture is a solution with thermodynamic properties analogous to those of a mixture of ideal gases. The enthalpy of solution is zero as is the volume change on mixing; the closer to zero the enthalpy of solution is, the more "ideal" the behavior of the...
is dependent on the vapor pressure of each chemical componentChemical compoundA chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
and the mole fraction of the component present in the solution.

Once the components in the solution have reached equilibrium
Vapor-liquid equilibrium
Vapor–liquid equilibrium is a condition where a liquid and its vapor are in equilibrium with each other, a condition or state where the rate of evaporation equals the rate of condensation on a molecular level such that there is no net vapor-liquid interconversion...
, the total vapor pressure p of the solution is:

and the individual vapor pressure for each component is

where
- pi is the partial pressurePartial pressureIn a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
of the component i in the mixture (in the solution) - p*i is the vapor pressureVapor pressureVapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
of the pure component i - xi is the mole fraction of the component i in the mixture (in the solution)
Consequently, as the number of components in a solution increases, the individual vapor pressures decrease, since the mole fraction of each component decreases with each additional component. If a pure solute which has zero vapor pressure (it will not evaporate) is dissolved in a solvent, the vapor pressure of the final solution will be lower than that of the pure solvent.
This law is strictly valid only under the assumption that the chemical interactions
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
between the two liquids is equal to the bonding within the liquids: the conditions of an ideal solution
Ideal solution
In chemistry, an ideal solution or ideal mixture is a solution with thermodynamic properties analogous to those of a mixture of ideal gases. The enthalpy of solution is zero as is the volume change on mixing; the closer to zero the enthalpy of solution is, the more "ideal" the behavior of the...
. Therefore, comparing actual measured vapor pressures to predicted values from Raoult's law allows information about the relative strength of bonding between liquids to be obtained. If the measured value of vapor pressure is less than the predicted value, fewer molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s have left the solution than expected. This is put down to the strength of bonding between the liquids being greater than the bonding within the individual liquids, so fewer molecules have enough energy to leave the solution. Conversely, if the vapor pressure is greater than the predicted value more molecules have left the solution than expected, due to the bonding between the liquids being less strong than the bonding within each.
The vapor pressure
Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
and composition in equilibrium with a solution can yield valuable information regarding the thermodynamic properties of the liquids involved. Raoult’s law relates the vapor pressure of components to the composition of the solution. The law assumes ideal behavior. It gives a simple picture of the situation just as the ideal gas law
Ideal gas law
The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law...
does. The ideal gas law is very useful as a limiting law. As the interactive forces between molecules and the volume of the molecules approaches zero, so the behavior of gases approach the behavior of the ideal gas.
Raoult’s law is similar in that it assumes that the physical properties of the components are identical. The more similar the components are, the more their behavior approaches that described by Raoult’s law. For example, if the two components differ only in isotopic
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
content, then the vapor pressure of each component will be equal to the vapor pressure of the pure substance
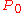 times the mole fraction in the solution. This is Raoult’s law.
times the mole fraction in the solution. This is Raoult’s law.Using the example of a solution of two liquids, A and B, if no other gases are present, then the total vapor pressure p above the solution is equal to the weighted sum of the "pure" vapor pressures of the two components, pA and pB. Thus the total pressure above solution of A and B would be
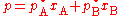
Derivation
We define an ideal solutionIdeal solution
In chemistry, an ideal solution or ideal mixture is a solution with thermodynamic properties analogous to those of a mixture of ideal gases. The enthalpy of solution is zero as is the volume change on mixing; the closer to zero the enthalpy of solution is, the more "ideal" the behavior of the...
as a solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
for which the chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
of component
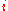 is:
is: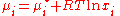 ,
,where µ*i is the chemical potential of pure i.
If the system is at equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
, then the chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
of the component i must be the same in the liquid solution and in the vapor
Vapor
A vapor or vapour is a substance in the gas phase at a temperature lower than its critical point....
above it. That is,
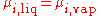
Assuming the liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
is an ideal solution, and using the formula for the chemical potential of a gas, gives:
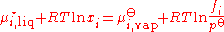
where ƒi is the fugacity
Fugacity
In chemical thermodynamics, the fugacity of a real gas is an effective pressure which replaces the true mechanical pressure in accurate chemical equilibrium calculations. It is equal to the pressure of an ideal gas which has the same chemical potential as the real gas. For example, nitrogen gas ...
of the vapor
Vapor
A vapor or vapour is a substance in the gas phase at a temperature lower than its critical point....
of
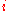 and
and  indicates reference state.
indicates reference state.The corresponding equation for pure
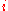 in equilibrium with its (pure) vapor is:
in equilibrium with its (pure) vapor is:
where * indicates the pure component.
Subtracting both equations gives us
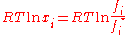
which re-arranges to
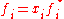
The fugacities
Fugacity
In chemical thermodynamics, the fugacity of a real gas is an effective pressure which replaces the true mechanical pressure in accurate chemical equilibrium calculations. It is equal to the pressure of an ideal gas which has the same chemical potential as the real gas. For example, nitrogen gas ...
can be replaced by simple pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
s if the vapor
Vapor
A vapor or vapour is a substance in the gas phase at a temperature lower than its critical point....
of the solution behaves ideally
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
i.e.
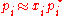
which is Raoult’s Law.
Ideal mixing
An ideal solution can be said to follow Raoult's Law but it must be kept in mind that in the strict sense ideal solutions do not exist. The fact that the vapor is taken to be ideal is the least of our worries. Interactions between gas molecules are typically quite small especially if the vapor pressures are low. The interactions in a liquid however are very strong. For a solution to be ideal we must assume that it does not matter whether a molecule A has another A as neighbor or a B molecule. This is only approximately true if the two species are almost identical chemically. We can see that from considering the Gibbs free energy change of mixing: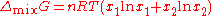
This is always negative, so mixing is spontaneous. However the expression is, apart from a factor –T, equal to the entropy of mixing. This leaves no room at all for an enthalpy effect and implies that ΔmixH must be equal to zero and this can only be if the interactions U between the molecules are indifferent.
It can be shown using the Gibbs–Duhem equation that if Raoult's law holds over the entire concentration range x = 0–1 in a binary solution then, for the second component, the same must also hold.
If the deviations from ideality are not too strong, Raoult's law will still be valid in a narrow concentration range when approaching x = 1 for the majority phase (the solvent). The solute will also show a linear limiting law but with a different coefficient. This law is known as Henry's law
Henry's law
In physics, Henry's law is one of the gas laws formulated by William Henry in 1803. It states that:An equivalent way of stating the law is that the solubility of a gas in a liquid at a particular temperature is proportional to the pressure of that gas above the liquid...
.
The presence of these limited linear regimes has been experimentally verified in a great number of cases.
In a perfectly ideal system, where ideal liquid and ideal vapor are assumed, a very useful equation emerges if Raoult's law is combined with Dalton's Law
Dalton's law
In chemistry and physics, Dalton's law states that the total pressure exerted by a gaseous mixture is equal to the sum of the partial pressures of each individual component in a gas mixture...
.
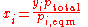
Non-ideal mixing
Raoult's Law may be adapted to non-ideal solutions by incorporating two factors that will account for the interactions between molecules of different substances. The first factor is a correction for gas non-ideality, or deviations from the ideal-gas law. It is called the fugacity coefficient ( ). The second, the activity coefficient (
). The second, the activity coefficient (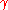 ), is a correction for interactions in the liquid phase between the different molecules.
), is a correction for interactions in the liquid phase between the different molecules.This modified or extended Raoult's law is then written:
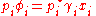
Real solutions

Negative deviation
When adhesive forces between molecules of A and B are greater than the cohesive force between A and A, or B and B, then the vapor pressure of the solution is less than the expected vapor pressure from Raoult's law. This is called a negative deviation from Raoult's law. These cohesive forces are lessened not only by dilution but also attraction between two molecules through formation of hydrogen bonds. This will further reduce the tendency of A and B to escape.
Positive deviation
When the cohesive forces between like molecules are greater than the adhesive forces, the dissimilarities of polarity or internal pressure will lead both components to escape solution more easily. Therefore, the vapor pressure will be greater than the expected from the Raoult's law, showing positive deviation. If the deviation is large, then the vapor pressure curve will show a maximum at a particular composition, e.g. benzene and ethyl alcohol, carbon disulfide and acetone, chloroform and ethanol.See also
- Atomic TheoryAtomic theoryIn chemistry and physics, atomic theory is a theory of the nature of matter, which states that matter is composed of discrete units called atoms, as opposed to the obsolete notion that matter could be divided into any arbitrarily small quantity...
- AzeotropeAzeotropeAn azeotrope is a mixture of two or more liquids in such a ratio that its composition cannot be changed by simple distillation. This occurs because, when an azeotrope is boiled, the resulting vapor has the same ratio of constituents as the original mixture....
- Dalton's lawDalton's lawIn chemistry and physics, Dalton's law states that the total pressure exerted by a gaseous mixture is equal to the sum of the partial pressures of each individual component in a gas mixture...
- DECHEMA modelDECHEMA modelThe DECHEMA model is a more general version of Raoult's law and under ideal conditions simplifies to Raoult's law. The DECHEMA model is a model for obtaining K values important in chemical engineering....
- Dühring's ruleDühring's ruleDühring's rule states that a linear relationship exists between the temperatures at which two solutions exert the same vapor pressure. The rule is often used to compare a pure liquid and a solution at a given concentration....
- Henry's lawHenry's lawIn physics, Henry's law is one of the gas laws formulated by William Henry in 1803. It states that:An equivalent way of stating the law is that the solubility of a gas in a liquid at a particular temperature is proportional to the pressure of that gas above the liquid...
- Köhler theoryKöhler theoryKöhler theory describes the process in which water vapor condenses and forms liquid cloud drops, and is based on equilibrium thermodynamics. It combines the Kelvin effect, which describes the change in saturation vapor pressure due to a curved surface, and Raoult's Law, which relates the saturation...
- SolubilitySolubilitySolubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...

