
Proislet Amyloid Polypeptide
Encyclopedia
Proislet Amyloid Polypeptide
(proIAPP,Proamylin, Amyloid Polypeptide Precursor, Proislet Protein)
Proislet amyloid polypeptide (proIAPP) is the protein precursor
for Islet amyloid polypeptide (IAPP, amylin) (Higham et al., 2001). ProIAPP is produced in the pancreatic beta cells (β-cells) as a 67 amino acid, 7404 Dalton pro-peptide and undergoes post-translational modifications to become the 37 amino acid IAPP hormone, also known as amylin
. IAPP is cosecreted with insulin
from the pancreatic β-cells in a ratio of approximately 100:1. Amylin plays a role in glycemic regulation by slowing gastric emptying and promoting satiety, thereby preventing post-prandial spikes in blood glucose levels.
 ProIAPP consists of 67 amino acids. The sequence (from N-terminus to C-terminus) is:
ProIAPP consists of 67 amino acids. The sequence (from N-terminus to C-terminus) is:
TPIESHQVEKRKCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTYGKRNAVEVLKREPLNYLPL (Higham et al., 2001).
Once produced by the β-cells, it undergoes proteolysis
. 11 amino acids are removed from the N-terminus by the enzyme proprotein convertase 2
(PC2) while 16 are removed from the C-terminus of the proIAPP molecule by proprotein convertase 1/3 (PC1/3) (Sanke, Bell, Sample, Rubenstein, & Steiner, 1988). At the C-terminus Carboxypeptidase E
then removes the terminal lysine
and arginine
residues (Marzban, Soukhatcheva, & Verchere, 2005). The terminal glycine
amino acid that results from this cleavage allows the enzyme peptidylglycine alpha-amidating monooxygenase (PAM) to add an amine
group. Finally, a disulfide
bond is formed between cysteine
residues numbers 2 and 7 (Roberts et al., 1989). After this the transformation from the precursor protein proIAPP to the biologically active IAPP is complete (IAPP sequence: KCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTY)(Higham et al., 2001).
. This is a condition wherein the body is unable to utilize insulin effectively, resulting in increased insulin production; since proinsulin
and proIAPP are cosecreted, this results in an increase in the production of proIAPP as well.
Although little is known about IAPP regulation, its connection to insulin indicates that regulatory mechanism that affect insulin also affect IAPP. Thus blood glucose levels play an important role in regulation of proIAPP synthesis.
formation, initiated by the aggregation of proIAPP, may contribute to this progressive loss of islet β-cells. It is thought that proIAPP forms the first granules that allow for IAPP to aggregate and form amyloid which may lead to amyloid-induced apoptosis
of β-cells.
IAPP is cosecreted with insulin. Insulin resistance in Type 2 diabetes produces a greater demand for insulin production which results in the secretion of proinsulin (Marzban, Rhodes, Haataja, Halban, & Verchere, 2006). ProIAPP is secreted simultaneously, however, the enzymes that convert these precursor molecules into insulin and IAPP, respectively, are not able to keep up with the high levels of secretion, ultimately leading to the accumulation of proIAPP.
In particular, the impaired processing of proIAPP that occurs at the N-terminal cleavage site is a key factor in the initiation of amyloid (Marzban et al., 2006). Post-translational modification of proIAPP occurs at both the carboxy terminus and the amino terminus, however, the processing of the amino terminus occurs later in the secretory pathway
. This might be one reason why it is more susceptible to impaired processing under conditions where secretion is in high demand (Marzban et al., 2005). Thus, the conditions of Type 2 diabetes—high glucose concentrations and increased secretory demand for insulin and IAPP—could lead to the impaired N-terminal processing of proIAPP. The unprocessed proIAPP can then serve as the granule upon which IAPP can accumulate and form amyloid (Paulsson et al., 2006).
The amyloid formation might be a major mediator of apoptosis, or programmed cell death, in the islet β-cells (Paulsson et al., 2006). Initially, the proIAPP aggregates inside the cell. The proIAPP acts as a seed, collecting IAPP from neighboring cells and forming an intracellular amyloid. As the amyloid grows, it spreads outside of the cell. The extracellular amyloid begins to excrete IAPP to other cells, inducing similar amyloid formation in other β-cells. The overall effect is an apoptosis cascade initiated by the influx of ions into the β-cells.
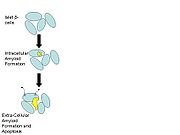 In summary, impaired N-terminal processing of proIAPP is an important factor initiating amyloid formation and β-cell death. These amyloid deposits are pathological characteristics of the pancreas
In summary, impaired N-terminal processing of proIAPP is an important factor initiating amyloid formation and β-cell death. These amyloid deposits are pathological characteristics of the pancreas
in Type 2 diabetes. However, it is still unclear as to whether amyloid formation is involved in or merely a consequence of type 2 diabetes (Marzban et al., 2006). Nevertheless it is clear that amyloid formation reduces working β-cells in patients with Type 2 diabetes. This suggests that repairing proIAPP processing may help to prevent β-cell death, thereby offering hope as a potential therapeutic approach for Type 2 diabetes.
(proIAPP,Proamylin, Amyloid Polypeptide Precursor, Proislet Protein)
Proislet amyloid polypeptide (proIAPP) is the protein precursor
Protein precursor
A protein precursor, also called a pro-protein or pro-peptide, is an inactive protein that can be turned into an active form by posttranslational modification. The name of the precursor for a protein is often prefixed by pro...
for Islet amyloid polypeptide (IAPP, amylin) (Higham et al., 2001). ProIAPP is produced in the pancreatic beta cells (β-cells) as a 67 amino acid, 7404 Dalton pro-peptide and undergoes post-translational modifications to become the 37 amino acid IAPP hormone, also known as amylin
Amylin
Amylin, or Islet Amyloid Polypeptide , is a 37-residue peptide hormone secreted by pancreatic β-cells at the same time as insulin .-Clinical significance:...
. IAPP is cosecreted with insulin
Insulin
Insulin is a hormone central to regulating carbohydrate and fat metabolism in the body. Insulin causes cells in the liver, muscle, and fat tissue to take up glucose from the blood, storing it as glycogen in the liver and muscle....
from the pancreatic β-cells in a ratio of approximately 100:1. Amylin plays a role in glycemic regulation by slowing gastric emptying and promoting satiety, thereby preventing post-prandial spikes in blood glucose levels.
Structure and Synthesis

TPIESHQVEKRKCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTYGKRNAVEVLKREPLNYLPL (Higham et al., 2001).
Once produced by the β-cells, it undergoes proteolysis
Proteolysis
Proteolysis is the directed degradation of proteins by cellular enzymes called proteases or by intramolecular digestion.-Purposes:Proteolysis is used by the cell for several purposes...
. 11 amino acids are removed from the N-terminus by the enzyme proprotein convertase 2
Proprotein convertase 2
Proprotein convertase 2 also known as prohormone convertase 2 or neuroendocrine convertase 2 is a serine protease and proprotein convertase PC2, like proprotein convertase 1 , is an enzyme responsible for the first step in the maturation of many neuroendocrine peptides from their precursors, such...
(PC2) while 16 are removed from the C-terminus of the proIAPP molecule by proprotein convertase 1/3 (PC1/3) (Sanke, Bell, Sample, Rubenstein, & Steiner, 1988). At the C-terminus Carboxypeptidase E
Carboxypeptidase E
Carboxypeptidase E, also known as carboxypeptidase H and enkephalin convertase, is an enzyme that in humans is encoded by the CPE gene....
then removes the terminal lysine
Lysine
Lysine is an α-amino acid with the chemical formula HO2CCH4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG....
and arginine
Arginine
Arginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during...
residues (Marzban, Soukhatcheva, & Verchere, 2005). The terminal glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
amino acid that results from this cleavage allows the enzyme peptidylglycine alpha-amidating monooxygenase (PAM) to add an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
group. Finally, a disulfide
Disulfide
In chemistry, a disulfide usually refers to the structural unit composed of a linked pair of sulfur atoms. Disulfide usually refer to a chemical compound that contains a disulfide bond, such as diphenyl disulfide, C6H5S-SC6H5....
bond is formed between cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
residues numbers 2 and 7 (Roberts et al., 1989). After this the transformation from the precursor protein proIAPP to the biologically active IAPP is complete (IAPP sequence: KCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTY)(Higham et al., 2001).
Regulation
Insulin and IAPP are regulated by similar factors since they share a common regulatory promoter sequence (Höppener, Ahrén, & Cornelius, 2000). One of the defining features of Type 2 diabetes is insulin resistanceInsulin resistance
Insulin resistance is a physiological condition where the natural hormone insulin becomes less effective at lowering blood sugars. The resulting increase in blood glucose may raise levels outside the normal range and cause adverse health effects, depending on dietary conditions. Certain cell types...
. This is a condition wherein the body is unable to utilize insulin effectively, resulting in increased insulin production; since proinsulin
Proinsulin
Proinsulin is the prohormone precursor to insulin made in the beta cells of the islets of Langerhans, specialized regions of the pancreas. In humans, proinsulin is encoded by the INS gene.- Synthesis and post-translational modification :...
and proIAPP are cosecreted, this results in an increase in the production of proIAPP as well.
Although little is known about IAPP regulation, its connection to insulin indicates that regulatory mechanism that affect insulin also affect IAPP. Thus blood glucose levels play an important role in regulation of proIAPP synthesis.
Role in Disease
ProIAPP has been linked to Type 2 diabetes and the loss of islet β-cells (Paulsson et al., 2005). Islet amyloidAmyloid
Amyloids are insoluble fibrous protein aggregates sharing specific structural traits. Abnormal accumulation of amyloid in organs may lead to amyloidosis, and may play a role in various neurodegenerative diseases.-Definition:...
formation, initiated by the aggregation of proIAPP, may contribute to this progressive loss of islet β-cells. It is thought that proIAPP forms the first granules that allow for IAPP to aggregate and form amyloid which may lead to amyloid-induced apoptosis
Apoptosis
Apoptosis is the process of programmed cell death that may occur in multicellular organisms. Biochemical events lead to characteristic cell changes and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation...
of β-cells.
IAPP is cosecreted with insulin. Insulin resistance in Type 2 diabetes produces a greater demand for insulin production which results in the secretion of proinsulin (Marzban, Rhodes, Haataja, Halban, & Verchere, 2006). ProIAPP is secreted simultaneously, however, the enzymes that convert these precursor molecules into insulin and IAPP, respectively, are not able to keep up with the high levels of secretion, ultimately leading to the accumulation of proIAPP.
In particular, the impaired processing of proIAPP that occurs at the N-terminal cleavage site is a key factor in the initiation of amyloid (Marzban et al., 2006). Post-translational modification of proIAPP occurs at both the carboxy terminus and the amino terminus, however, the processing of the amino terminus occurs later in the secretory pathway
Secretory pathway
The secretory pathway is a series of steps a cell uses to move proteins out of the cell; a process known as secretion. The path of a protein destined for secretion has its origins in the rough endoplasmic reticulum, a membrane-bound compartment in the cell...
. This might be one reason why it is more susceptible to impaired processing under conditions where secretion is in high demand (Marzban et al., 2005). Thus, the conditions of Type 2 diabetes—high glucose concentrations and increased secretory demand for insulin and IAPP—could lead to the impaired N-terminal processing of proIAPP. The unprocessed proIAPP can then serve as the granule upon which IAPP can accumulate and form amyloid (Paulsson et al., 2006).
The amyloid formation might be a major mediator of apoptosis, or programmed cell death, in the islet β-cells (Paulsson et al., 2006). Initially, the proIAPP aggregates inside the cell. The proIAPP acts as a seed, collecting IAPP from neighboring cells and forming an intracellular amyloid. As the amyloid grows, it spreads outside of the cell. The extracellular amyloid begins to excrete IAPP to other cells, inducing similar amyloid formation in other β-cells. The overall effect is an apoptosis cascade initiated by the influx of ions into the β-cells.

Pancreas
The pancreas is a gland organ in the digestive and endocrine system of vertebrates. It is both an endocrine gland producing several important hormones, including insulin, glucagon, and somatostatin, as well as a digestive organ, secreting pancreatic juice containing digestive enzymes that assist...
in Type 2 diabetes. However, it is still unclear as to whether amyloid formation is involved in or merely a consequence of type 2 diabetes (Marzban et al., 2006). Nevertheless it is clear that amyloid formation reduces working β-cells in patients with Type 2 diabetes. This suggests that repairing proIAPP processing may help to prevent β-cell death, thereby offering hope as a potential therapeutic approach for Type 2 diabetes.
See also
- islet amyloid polypeptide (IAPP)
- Diabetes mellitusDiabetes mellitusDiabetes mellitus, often simply referred to as diabetes, is a group of metabolic diseases in which a person has high blood sugar, either because the body does not produce enough insulin, or because cells do not respond to the insulin that is produced...
- amyloidAmyloidAmyloids are insoluble fibrous protein aggregates sharing specific structural traits. Abnormal accumulation of amyloid in organs may lead to amyloidosis, and may play a role in various neurodegenerative diseases.-Definition:...
- islets of LangerhansIslets of LangerhansThe islets of Langerhans are the regions of the pancreas that contain its endocrine cells. Discovered in 1869 by German pathological anatomist Paul Langerhans at the age of 22, the islets of Langerhans constitute approximately 1 to 2% of the mass of the pancreas...
- proprotein convertase 2Proprotein convertase 2Proprotein convertase 2 also known as prohormone convertase 2 or neuroendocrine convertase 2 is a serine protease and proprotein convertase PC2, like proprotein convertase 1 , is an enzyme responsible for the first step in the maturation of many neuroendocrine peptides from their precursors, such...
(PC2)
- proprotein covertase 1/3 (PC1/3)
- carboxypeptidase ECarboxypeptidase ECarboxypeptidase E, also known as carboxypeptidase H and enkephalin convertase, is an enzyme that in humans is encoded by the CPE gene....
- peptidylglycine alpha-amidating monooxygenase (PAM)

