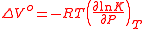Pressure jump is a technique used in the study of
chemical kineticsChemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
. It involves making rapid changes to the pressure of an experimental system and observing the return to
equilibriumIn a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
or
steady stateIn chemistry, a steady state is a situation in which all state variables are constant in spite of ongoing processes that strive to change them. For an entire system to be at steady state, i.e. for all state variables of a system to be constant, there must be a flow through the system...
. This allows the study of the shift in equilibrium of reactions that equilibrate in periods between milliseconds to hours (or longer),
[This contrasts with temperature jump]Temperature jump is a technique used in the study of chemical kinetics. It usually involves the discharging of a capacitor through a small volume Temperature jump is a technique used in the study of chemical kinetics. It usually involves the discharging of a capacitor (in the kV range) through a...
in which cooling curves typically limit the time window to a minute or so. these changes often being observed using absorption spectroscopy, or
fluorescence spectroscopyFluorescence spectroscopy aka fluorometry or spectrofluorometry, is a type of electromagnetic spectroscopy which analyzes fluorescence from a sample. It involves using a beam of light, usually ultraviolet light, that excites the electrons in molecules of certain compounds and causes them to emit...
though other spectroscopic techniques such as
CDCircular dichroism refers to the differential absorption of left and right circularly polarized light. This phenomenon was discovered by Jean-Baptiste Biot, Augustin Fresnel, and Aimé Cotton in the first half of the 19th century. It is exhibited in the absorption bands of optically active chiral...
,
FTIRFourier transform infrared spectroscopy is a technique which is used to obtain an infrared spectrum of absorption, emission, photoconductivity or Raman scattering of a solid, liquid or gas. An FTIR spectrometer simultaneously collects spectral data in a wide spectral range...
or
NMRNuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation...
can also used.
Historically, pressure jumps were limited to one direction. Most commonly fast drops in pressure were achieved by using a quick release valve or a fast burst membrane. Modern equipment can achieve pressure changes in both directions using either double reservoir arrangements (good for large changes in pressure) or pistons operated by piezoelectric actuators (often faster than valve based approaches). Ultra fast pressure drops can be achieved using electrically disintegrated burst membranes. The ability to automatically repeat measurements and average the results is useful since the reaction amplitudes are often small.
The fractional extent of the reaction (i.e. the percentage change in concentration of a measurable species) depends on the molar volume change (ΔV°) between the reactants and products and the equilibrium position. If K is the equilibrium constant and P is the pressure then the volume change is given by:
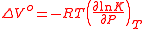
where R is the universal gas constantThe gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
and T is the absolute temperatureThermodynamic temperature is the absolute measure of temperature and is one of the principal parameters of thermodynamics. Thermodynamic temperature is an "absolute" scale because it is the measure of the fundamental property underlying temperature: its null or zero point, absolute zero, is the...
. The volume change can thus be understood to be the pressure dependency of the change in Gibbs free energyIn thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
associated with the reaction.
When a single step in a reaction is perturbed in a pressure jump experiment, the reaction follows a single exponential decay function with the reciprocal time constantIn physics and engineering, the time constant, usually denoted by the Greek letter \tau , is the risetime characterizing the response to a time-varying input of a first-order, linear time-invariant system.Concretely, a first-order LTI system is a system that can be modeled by a single first order...
(1/τ) equal to the sum of the forward and reverse intrinsic rate constants. In more complex reaction networks, when multiple reaction steps are perturbed, then the reciprocal time constants are given by the eigenvalues of the characteristic rate equations. The ability to observe intermediate steps in a reaction pathway is one of the attractive features of this technology.
The source of this article is
wikipedia, the free encyclopedia. The text of this article is licensed under the
GFDL.