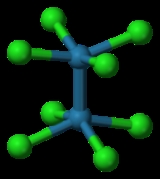
Potassium octachlorodimolybdate
Encyclopedia
Potassium octachlorodimolybdate is the inorganic compound with the formula K4Mo2Cl8. This red-coloured salt consists of potassium
cations and the octachlorodimolybdate anion, Mo2Cl84−. The anion is of historic interest because it was one of the earliest illustrations of a quadruple bond
ing. The salt is usually obtained as the dihydrate.
The compound is prepared in two steps from molybdenum hexacarbonyl
:
-3d-balls.png) The reaction of the acetate with HCl was first described as providing trimolybdenum compounds, but subsequent crystallographic analysis confirmed that the product contains the Mo2Cl84− ion with D4h symmetry. The Mo---Mo distance is 2.14 Å.
The reaction of the acetate with HCl was first described as providing trimolybdenum compounds, but subsequent crystallographic analysis confirmed that the product contains the Mo2Cl84− ion with D4h symmetry. The Mo---Mo distance is 2.14 Å.
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
cations and the octachlorodimolybdate anion, Mo2Cl84−. The anion is of historic interest because it was one of the earliest illustrations of a quadruple bond
Quadruple bond
A quadruple bond is a type of chemical bond between two atoms involving eight electrons. This bond is an extension of the more familiar types double bonds and triple bonds. Stable quadruple bonds are most common among the middle members transition metal elements such rhenium, tungsten, molybdenum...
ing. The salt is usually obtained as the dihydrate.
The compound is prepared in two steps from molybdenum hexacarbonyl
Molybdenum hexacarbonyl
Molybdenum hexacarbonyl is the chemical compound with the formula Mo6. This colorless solid, like its chromium and tungsten analogues, is noteworthy as a volatile, air-stable derivative of a metal in its zero oxidation state.-Structure and properties:Mo6 adopts an octahedral geometry consisting...
:
- 2 Mo(CO)6 + 4 HO2CCH3Acetic acidAcetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
→ Mo2(O2CCH3)4 + 2 H2 + 12 CO - Mo2(O2CCH3)4 + 4 HCl + 4 KCl → K4Mo2Cl8 + 4 HO2CCH3
-3d-balls.png)

