PSRK
Encyclopedia
PSRK
is an estimation method for the calculation of phase equilibria of mixtures of chemical components. The original goal for the development of this method was to enable the estimation of properties of mixtures which contain supercritical components. This class of substances cannot be predicted with established models, for example UNIFAC
.
. This is a class of prediction methods which combines equations of state (mostly cubic) with activity coefficient
models based on group contributions
, such as UNIFAC. The activity coefficient model is used to adapt the equation of state parameters for mixtures by a so called mixing rule.
The use of an equation of state introduces all thermodynamic relations defined for equations of state into the PRSK model. This allows the calculation of densities
, enthalpies
, heat capacities
, and other properties.

The original α-function has been replaced by the function of Mathias-Copeman
.

The parameters of the Mathias-Copeman equation are fitted to experimental vapor pressure data of pure components and provide a better description of the vapor pressure than the original relation. The form of the equation is chosen as it can be reduced to the original Soave form by setting the parameters c2 und c3 to zero. Additionally, the parameter c1 can be obtained from the acentric factor
, using the relation:

This may be performed if no fitted Mathias-Copeman parameter is available.

and

Where the parameters ai and bi are those of the pure substances, their mole fractions are given by xi and the excess Gibbs energy gE. The excess Gibbs energy is calculated by a slightly modified UNIFAC model.
The integrity of the model can be improved if the acentric factor is replaced by Mathias-Copeman constants which have been fitted to experimental vapor pressure data of pure components.
The mixing rule uses UNIFAC which needs a variety of UNIFAC-specific parameters. Aside from some model constants, the most important parameters are the group interaction parameters --- these are obtained from parametric fits to experimental vapor-liquid equilibria of mixtures.
Hence, for high-quality model parameters experimental data (pure component vapor pressures and VLE of mixtures) are needed. These are normally provided by factual data banks like the Dortmund Data Bank
which has been the base for the PSRK development.
In few cases additionally needed data have been determined experimentally if no data have been available from other sources.
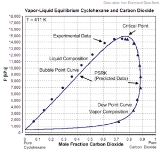
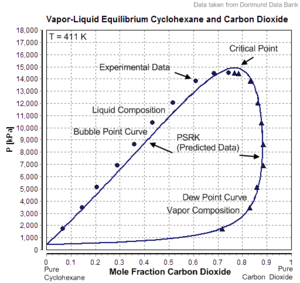 However, the mixture has to be subcritical. In the given example carbon dioxide is the supercritical component with Tc=304.19 K and Pc=7475 kPa. The critical point of the mixture lies at T=411 K und P≈15000 kPa. The composition of the mixture is near 78 mole% carbon dioxide und 22 mole% cyclohexane.
However, the mixture has to be subcritical. In the given example carbon dioxide is the supercritical component with Tc=304.19 K and Pc=7475 kPa. The critical point of the mixture lies at T=411 K und P≈15000 kPa. The composition of the mixture is near 78 mole% carbon dioxide und 22 mole% cyclohexane.
PSRK describes this binary mixture quite well, the dew point curve as well as the bubble point
curve and the critical point of the mixture.
is an estimation method for the calculation of phase equilibria of mixtures of chemical components. The original goal for the development of this method was to enable the estimation of properties of mixtures which contain supercritical components. This class of substances cannot be predicted with established models, for example UNIFAC
UNIFAC
The UNIversal Functional Activity Coefficient method is a semi-empirical system for the prediction of non-electrolyte activity estimation in non-ideal mixtures. UNIFAC uses the functional groups present on the molecules that make up the liquid mixture to calculate activity coefficients...
.
Principle
PSRK is a group contribution equation of stateEquation of state
In physics and thermodynamics, an equation of state is a relation between state variables. More specifically, an equation of state is a thermodynamic equation describing the state of matter under a given set of physical conditions...
. This is a class of prediction methods which combines equations of state (mostly cubic) with activity coefficient
Activity coefficient
An activity coefficient is a factor used in thermodynamics to account for deviations from ideal behaviour in a mixture of chemical substances. In an ideal mixture, the interactions between each pair of chemical species are the same and, as a result, properties of the mixtures can be expressed...
models based on group contributions
Group contribution method
A group contribution method is a technique to estimate and predict thermodynamic and other properties from molecular structures.- Introduction :In today's chemical processes hundreds of thousands of components are used...
, such as UNIFAC. The activity coefficient model is used to adapt the equation of state parameters for mixtures by a so called mixing rule.
The use of an equation of state introduces all thermodynamic relations defined for equations of state into the PRSK model. This allows the calculation of densities
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
, enthalpies
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
, heat capacities
Heat capacity
Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
, and other properties.
Equations
As stated previously, the PSRK model is based on a combination of the Soave-Redlich-Kwong equation of state with a mixing rule whose parameters are determined by the UNIFAC method.Equation of State
The equation of state of Soave is defined as follows:
The original α-function has been replaced by the function of Mathias-Copeman
.

The parameters of the Mathias-Copeman equation are fitted to experimental vapor pressure data of pure components and provide a better description of the vapor pressure than the original relation. The form of the equation is chosen as it can be reduced to the original Soave form by setting the parameters c2 und c3 to zero. Additionally, the parameter c1 can be obtained from the acentric factor
Acentric factor
The acentric factor \omega is a conceptual number introduced by Pitzer in 1955, proven to be very useful in the description of matter. It has become a standard for the phase characterization of single & pure components...
, using the relation:

This may be performed if no fitted Mathias-Copeman parameter is available.
Mixing Rule
The PSRK mixing rule calculates the parameters a and b of the equation of state by
and

Where the parameters ai and bi are those of the pure substances, their mole fractions are given by xi and the excess Gibbs energy gE. The excess Gibbs energy is calculated by a slightly modified UNIFAC model.
Model Parameters
For the equation of state PSRK needs the critical temperature and pressure, additionally at a minimum the acentric factor for all pure components in the considered mixture is also required.The integrity of the model can be improved if the acentric factor is replaced by Mathias-Copeman constants which have been fitted to experimental vapor pressure data of pure components.
The mixing rule uses UNIFAC which needs a variety of UNIFAC-specific parameters. Aside from some model constants, the most important parameters are the group interaction parameters --- these are obtained from parametric fits to experimental vapor-liquid equilibria of mixtures.
Hence, for high-quality model parameters experimental data (pure component vapor pressures and VLE of mixtures) are needed. These are normally provided by factual data banks like the Dortmund Data Bank
Dortmund Data Bank
The Dortmund Data Bank is a factual data bank for thermodynamic and thermophysical data. Its main usage is the data supply for process simulation where experimental data are the basis for the design, analysis, synthesis, and optimization of chemical processes...
which has been the base for the PSRK development.
In few cases additionally needed data have been determined experimentally if no data have been available from other sources.
Example Calculation
The prediction of a vapor-liquid equilibrium is successful even in mixtures containing supercritical components.
PSRK describes this binary mixture quite well, the dew point curve as well as the bubble point
Bubble point
When heating a liquid consisting of two or more components, the bubble point is the point where first bubble of vapor is formed. Given that vapor will probably have a different composition than the liquid, the bubble point at different compositions are useful data when designing distillation...
curve and the critical point of the mixture.
Model Weaknesses
In a PSRK follow-up work some model weaknesses are quoted:- The gradient of the Mathias-Copeman α-function is without any thermodynamic background and, if extrapolated to higher temperatures, the described vapor pressure curve tends to diverge.
- The Soave-Redlich-Kwong equation of state describes the vapor densities of pure components and mixtures quite well but the deviations of the liquid density prediction are high.
- For the VLE prediction of mixtures with components which have very differing sizes (e. g. EthanolEthanolEthanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
, C2H6O, and EicosaneEicosaneIcosane is an alkane with the chemical formula C20H42. It has 366,319 constitutional isomers....
, C20H44) larger systematic errors are found. - Heats of mixing and activity coefficients at infinite dilution are predicted poorly.
External links
- Short PSRK description from the developers
- UNIFAC Consortium at the Carl von Ossietzky University Oldenburg (develops the PSRK model since 2005)

