
Nicotine dehydrogenase
Encyclopedia
In enzymology, a nicotine dehydrogenase is an enzyme
that catalyzes
the chemical reaction
-nicotine + acceptor + H2O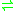 (S)-6-hydroxynicotine + reduced acceptor
(S)-6-hydroxynicotine + reduced acceptor
The 3 substrates
of this enzyme are (S)-nicotine, acceptor
, and H2O
, whereas its two products
are (S)-6-hydroxynicotine and reduced acceptor.
This enzyme belongs to the family of oxidoreductase
s, specifically those acting on the CH-NH group of donors with other acceptors. The systematic name of this enzyme class is nicotine:acceptor 6-oxidoreductase (hydroxylating). Other names in common use include nicotine oxidase, D-nicotine oxidase, nicotine:(acceptor) 6-oxidoreductase (hydroxylating), and L-nicotine oxidase. It has 2 cofactors
: metal
, and FMN
.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
-nicotine + acceptor + H2O
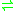 (S)-6-hydroxynicotine + reduced acceptor
(S)-6-hydroxynicotine + reduced acceptorThe 3 substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
of this enzyme are (S)-nicotine, acceptor
Electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process....
, and H2O
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, whereas its two products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
are (S)-6-hydroxynicotine and reduced acceptor.
This enzyme belongs to the family of oxidoreductase
Oxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule to another...
s, specifically those acting on the CH-NH group of donors with other acceptors. The systematic name of this enzyme class is nicotine:acceptor 6-oxidoreductase (hydroxylating). Other names in common use include nicotine oxidase, D-nicotine oxidase, nicotine:(acceptor) 6-oxidoreductase (hydroxylating), and L-nicotine oxidase. It has 2 cofactors
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
: metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
, and FMN
FMN
FMN may refer to several things:* Flavin mononucleotide.* Forsvarsministeriet, the Danish Ministry of Defence.* Four Corners Regional Airport outside Farmington, New Mexico, its IATA airport code.* Fluid Music....
.

