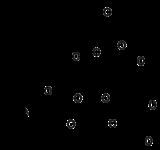
Mitemcinal
Encyclopedia
Mitemcinal is a motilin
agonist
derived from the macrolide antibiotic, erythromycin
. It was discovered in the labs of Chugai Pharma
. Mitemcinal is orally administered and it is believed to have strong promotility (or prokinetic) effects. Promotility drugs relieve symptoms of reflux by speeding the clearance of acid from the oesophagus and stomach. The parent compound, erythromycin, has these characteristics, but mitemcinal lacks the antibiotic properties of erythromycin.
Presently, erythromycin is commonly used off-label for gastric motility indications such as gastroparesis
. If mitemcinal can be shown to be as effective a promotility agent, it would represent a significant advance in the GI field as treatment with this drug would not carry the risk of unintentional selection of antibiotic-resistant bacteria. This concept greatly intrigued Chugai, and the company pursued it as an ideal candidate for a long-term prokinetic agent to treat lower gastrointestinal deficiencies including gastroparesis.
The Phase II trials did not detect an increase in gastric emptying among the population of diabetic gastroparesis patients above placebo. Thus, although Chugai was able to demonstrate the required safety of the compound, development of this drug has stalled due to a lack of convincing efficacy data.
Motilin
Motilin is a 22-amino acid polypeptide hormone in the motilin family that, in humans, is encoded by the MLN gene.Motilin is secreted by endocrine M cells that are numerous in crypts of the small intestine, especially in the duodenum and jejunum. Based on amino acid sequence, motilin is unrelated...
agonist
Agonist
An agonist is a chemical that binds to a receptor of a cell and triggers a response by that cell. Agonists often mimic the action of a naturally occurring substance...
derived from the macrolide antibiotic, erythromycin
Erythromycin
Erythromycin is a macrolide antibiotic that has an antimicrobial spectrum similar to or slightly wider than that of penicillin, and is often used for people who have an allergy to penicillins. For respiratory tract infections, it has better coverage of atypical organisms, including mycoplasma and...
. It was discovered in the labs of Chugai Pharma
Chugai Pharmaceutical Co.
Chugai Pharmaceutical Co., Ltd. is a subsidiary drug manufacturer operating in Japan controlled by Hoffmann–La Roche, which owns 52% of the company...
. Mitemcinal is orally administered and it is believed to have strong promotility (or prokinetic) effects. Promotility drugs relieve symptoms of reflux by speeding the clearance of acid from the oesophagus and stomach. The parent compound, erythromycin, has these characteristics, but mitemcinal lacks the antibiotic properties of erythromycin.
Presently, erythromycin is commonly used off-label for gastric motility indications such as gastroparesis
Gastroparesis
Gastroparesis, also called delayed gastric emptying, is a medical condition consisting of a paresis of the stomach, resulting in food remaining in the stomach for a longer period of time than normal. Normally, the stomach contracts to move food down into the small intestine for digestion. The...
. If mitemcinal can be shown to be as effective a promotility agent, it would represent a significant advance in the GI field as treatment with this drug would not carry the risk of unintentional selection of antibiotic-resistant bacteria. This concept greatly intrigued Chugai, and the company pursued it as an ideal candidate for a long-term prokinetic agent to treat lower gastrointestinal deficiencies including gastroparesis.
The Phase II trials did not detect an increase in gastric emptying among the population of diabetic gastroparesis patients above placebo. Thus, although Chugai was able to demonstrate the required safety of the compound, development of this drug has stalled due to a lack of convincing efficacy data.

