
L-amino-acid dehydrogenase
Encyclopedia
In enzymology, a L-amino-acid dehydrogenase is an enzyme
that catalyzes
the chemical reaction
The 3 substrates
of this enzyme are L-amino acid, H2O
, and NAD+
, whereas its 4 products
are 2-oxo acid, NH3
, NADH
, and H+
.
This enzyme belongs to the family of oxidoreductase
s, specifically those acting on the CH-NH2 group of donors with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is L-amino-acid:NAD+ oxidoreductase (deaminating).
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- an L-amino acid + H2O + NAD+
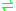 a 2-oxo acid + NH3 + NADH + H+
a 2-oxo acid + NH3 + NADH + H+
The 3 substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
of this enzyme are L-amino acid, H2O
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, and NAD+
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide, abbreviated NAD, is a coenzyme found in all living cells. The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide.In metabolism, NAD is involved...
, whereas its 4 products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
are 2-oxo acid, NH3
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
, NADH
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide, abbreviated NAD, is a coenzyme found in all living cells. The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide.In metabolism, NAD is involved...
, and H+
Hydrogen ion
Hydrogen ion is recommended by IUPAC as a general term for all ions of hydrogen and its isotopes.Depending on the charge of the ion, two different classes can be distinguished: positively charged ions and negatively charged ions....
.
This enzyme belongs to the family of oxidoreductase
Oxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule to another...
s, specifically those acting on the CH-NH2 group of donors with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is L-amino-acid:NAD+ oxidoreductase (deaminating).

